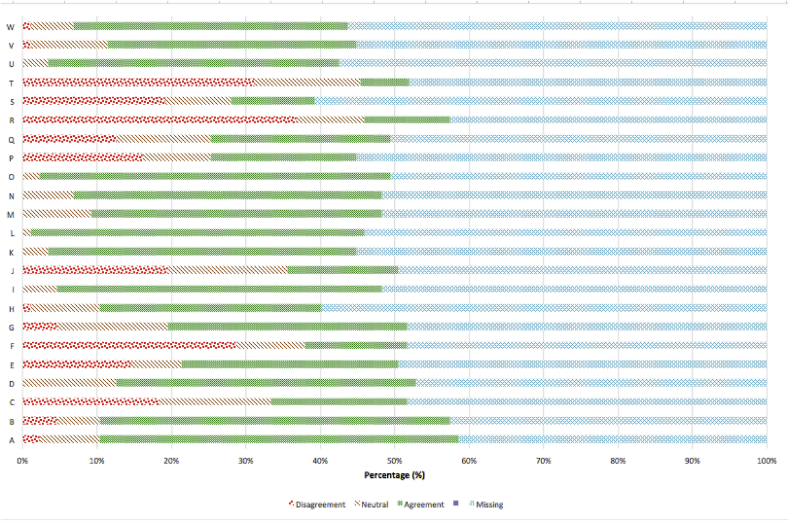
Fig. 3.
Respondents' agreement and disagreement on statements related to participation in clinical research.
A I gained something positive from participating.
B Knowing what I know now, I would participate in clinical research if given the opportunity.
C The research raised emotional issues for me that I had not expected.
D I gained insight about my experiences through research participation.
E The research made me think about things I didn't want to think about.
F I found the questions too personal.
G I found participating in the clinical research personally meaningful.
H I believe the clinical research results will be useful to others.
I I trusted that my replies would be kept private.
J I experienced intense emotions during the research session and/or parts of the study.
K I think clinical research is for a good cause.
L I was treated with respect and dignity when I participated in clinical research.
M I found participating in clinical research beneficial to me.
N I was glad to be asked to participate.
O I like the idea that I contributed to science.
P I was emotional during the research session.
Q I felt I could stop participating at any time.
R I found participating boring.
S The study procedures took too long.
T Participating in clinical research was inconvenient for me.
U Participation was a choice I freely made
V Had I known in advance what participating would be like I still would have agreed to participate.
W I understood the consent form.