Abstract
A 71-year-old man with castration-resistant prostate cancer (CRPC) demonstrated a flare phenomenon on 99mTc-MDP and CT after 10 weeks of enzalutamide. Prostate-specific membrane antigen (PSMA)-targeted 18F-DCFPyL PET/CT demonstrated minimal uptake at sites of baseline bone and lymph node disease with increasing uptake at sites of osseous disease following therapy. While this is likely related in part to decreased androgen receptor activity and a consequent increase in PSMA expression, other mechanisms (neovascularization, cell infiltration from the bone repair process, osteoblastic turnover, or minimal radiotracer impurity) may also be involved in causing the increased 18F-DCFPyL uptake at sites of osseous flare.
Figure 1.
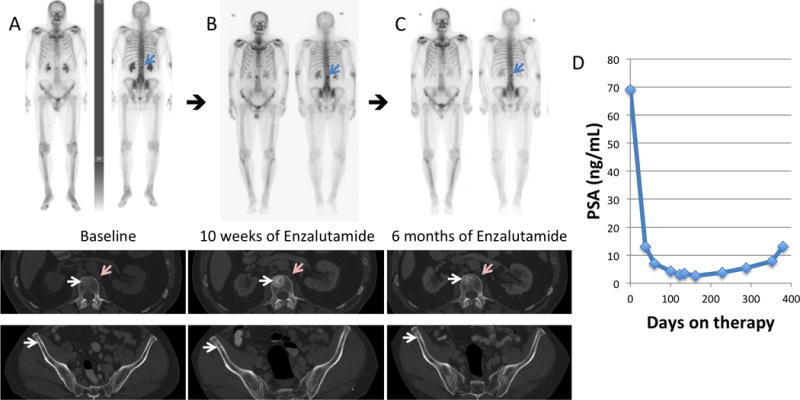
(A) 99mTc-MDP skeletal scintigraphy and diagnostic CT performed at baseline, (B) after 10 weeks, and (C) after 6 months of enzalutamide in a 71-year-old man with known CRPC, who had previously been naïve to treatment with a second generation anti-androgen. Osseous disease at baseline became more sclerotic/conspicuous on the follow-up bone scan and CT after 10 weeks of therapy (white arrows) correlating with decreasing PSA (D). The 6 month follow-up bone scan and CT confirms a flare phenomenon, similar to that described in the literature1–4. There was also lymph node disease on the baseline diagnostic CT (pink arrows) that improved on the diagnostic CT scans obtained after 10 weeks and 6 months of therapy. PSA nadir was seen at approximately 6 months post-initiation of enzalutamide, and therapy was discontinued at 1 year due to progression.
Figure 2.
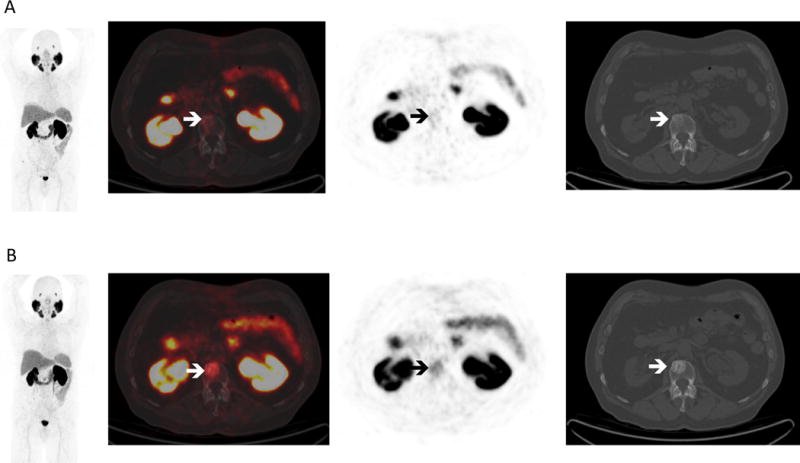
(A) PSMA-targeted 18F-DCFPyL PET/CT at baseline and (B) after 10 weeks of enzalutamide in the same man. At baseline, there was minimal to no significant radiotracer uptake at the sites of bone and lymph node disease seen on the bone scan and diagnostic CT. After 10 weeks of therapy there was significant increase in radiotracer uptake correlating with increased sclerosis in L2 (white arrows). 18F-DCFPyL is known to have high uptake at sites of metastatic prostate cancer5,6; however, this case shows limited 18F-DCFPyL uptake at sites of disease, underscoring the fact that PSMA expression in prostate cancer cells is variable7,8 and uptake of PSMA-targeted agents may reflect this variability.
Figure 3.
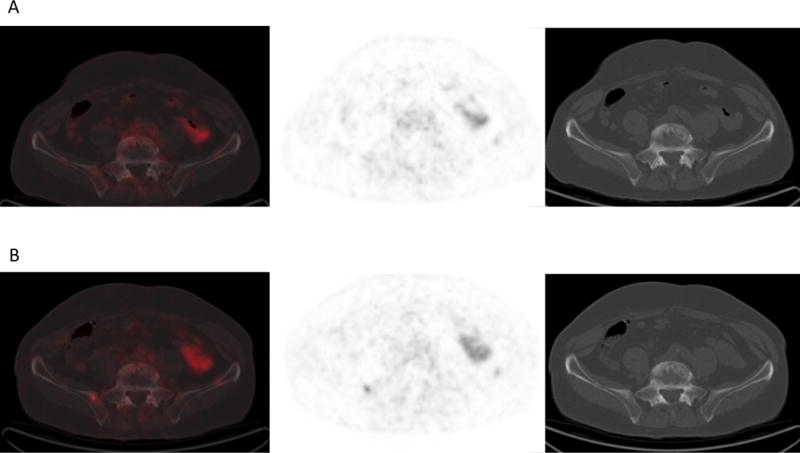
(A) 18F-DCFPyL PET/CT at baseline and (B) after 10 weeks of therapy in the same man show mild increased radiotracer uptake correlating with increased sclerosis in a small site of disease in the right iliac bone adjacent to the sacroiliac joint.
Figure 4.
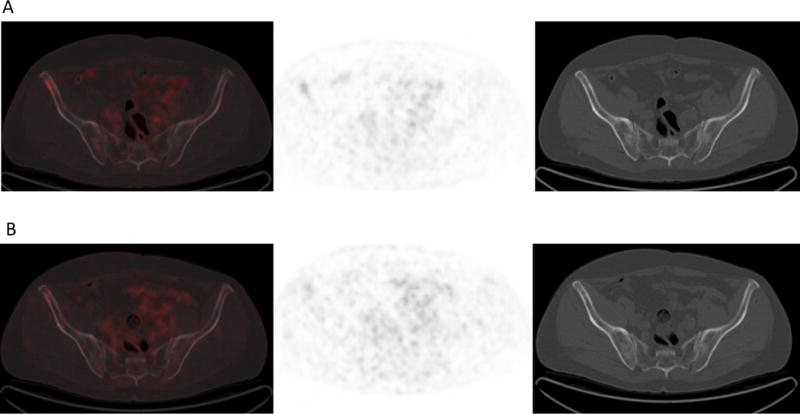
(A) 18F-DCFPyL PET/CT at baseline and (B) after 10 weeks of therapy in the same man show mildly avid predominantly lytic disease in the right iliac bone anteriorly at baseline that decreased slightly following therapy. This site of disease showed minimal increased sclerosis following therapy.
Figure 5.
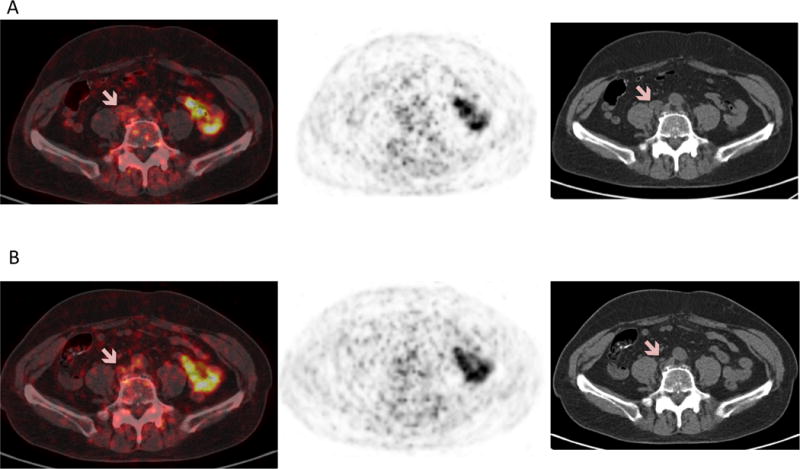
(A) 18F-DCFPyL PET/CT at baseline and (B) after 10 weeks of therapy in the same man showed no significant radiotracer uptake associated with lymph nodes seen on CT either at baseline or following therapy (pink arrows). Although it has been suggested that exposure to androgen deprivation therapy can increase PSMA expression and radiotracer uptake on PSMA targeted PET/CT, this was not seen here at sites of lymph node disease. Also, literature shows PET detects up-regulation of PSMA in response to androgen deprivation therapy9,10; however the only sites of increasing radiotracer uptake were at sites of osseous flare. Since 18F-DCFPyL uptake can occur at sites of benign osseous disease such as Paget disease11 or metastatic prostate cancer to bone with a predominantly osteoblastic reaction and few viable tumor cells12, in addition to changes in androgen receptor signaling, possible hypotheses for uptake at sites of osseous flare include: neovascularization, cell infiltration from bone repair, or osteoblastic turnover.
Footnotes
Conflict of interest disclosure statement: MGP is a co-inventor on a U.S. patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. SPR and MGP receive research support from Progenics Pharmaceuticals, the licensee of 18F-DCFPyL. No other potential conflict of interest relevant to this article was reported.
Contributor Information
Katherine A. Zukotynski, McMaster University, Hamilton, ON.
John Valliant, McMaster University, Hamilton, ON.
François Bénard, University of British Columbia, Vancouver, BC.
Steven P. Rowe, Johns Hopkins University, Baltimore, MD.
Chun K. Kim, Harvard Medical School, Boston, MA.
Martin G. Pomper, Johns Hopkins University, Baltimore, MD.
Steve Y. Cho, University of Wisconsin, Madison, WI.
References
- 1.Coleman RE, Mashiter G, Whitaker KB, et al. Bone Scan Flare Predicts Successful Systemic Therapy for Bone Metastases. J Nucl Med. 1988;29:1354–1359. [PubMed] [Google Scholar]
- 2.Cook GJR, Venkitaraman R, Sohaib AS, et al. The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur J Nucl Med Mol Imaging. 2011;38:7–13. doi: 10.1007/s00259-010-1576-0. [DOI] [PubMed] [Google Scholar]
- 3.Pollen JJ, Witztum KF, Ashburn WL. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am J Roentgenol. 1984;142:773–776. doi: 10.2214/ajr.142.4.773. [DOI] [PubMed] [Google Scholar]
- 4.Messiou C, Cook G, Reid AHM. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiologica. 2011;52:557–561. doi: 10.1258/ar.2011.100342. [DOI] [PubMed] [Google Scholar]
- 5.Wright GL, Jr, Grob BM, Haley C, et al. Up-regulation of prostate-specific membrane antigen after androgen deprivation therapy. Urology. 1996;48(2):326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 6.Israeli RS, Powell CT, Corr JG, et al. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–1811. [PubMed] [Google Scholar]
- 7.Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intra- epithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Mannweiler S, Amersdorfer P, Trajanoski S, et al. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 9.Evans MJ, Smith-Jones PM, Wongvipat J, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci USA. 2011;108(23):9578–9582. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meller B, Bremmer F, Sahlmann CO, et al. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Research. 2015;5:66–76. doi: 10.1186/s13550-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe SP, Deville C, Paller C, et al. Uptake of 18F-DCFPyL in Paget’s Disease of Bone, an Important Potential Pitfall in Clinical Interpretation of PSMA PET Studies. Tomography. 2015;1(2):81–84. doi: 10.18383/j.tom.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe SP, Macura KJ, Ciarallo A, et al. Comparison of Prostate-Specific Membrane Antigen-Based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Naïve and Castration-Resistant Metastatic Prostate Cancer. J Nucl Med. 2016;57:46–53. doi: 10.2967/jnumed.115.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]


