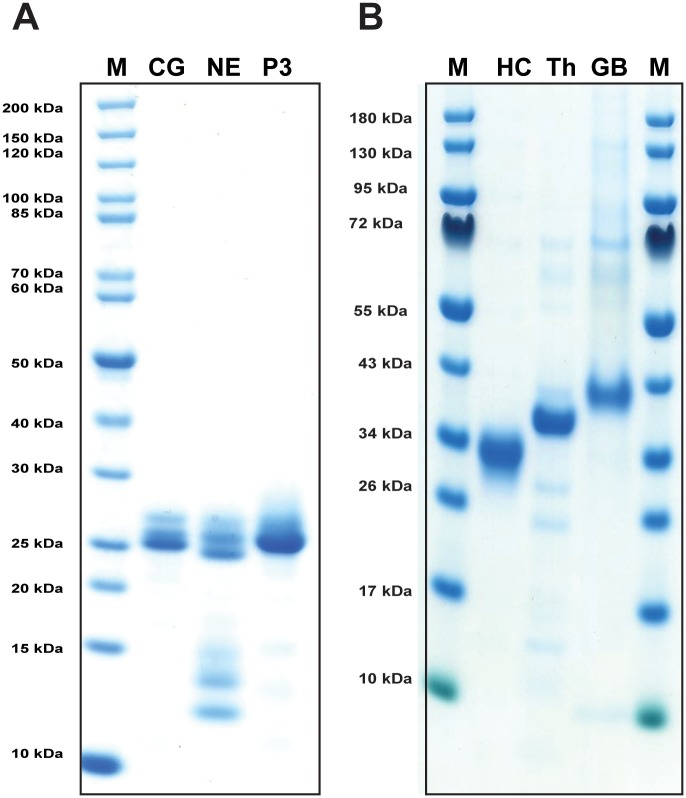
Fig 1. Analysis of the human cathepsin G preparation used in the determination of the extended cleavage specificity.
The enzyme had previously been purified from blood neutrophils and the purity of the preparation was determined by separation on SDS-PAGE and visualized with Coomassie Brilliant Blue staining. In panel A commercial preparations of two additional neutrophil proteases N-elastase (NE) and proteinase 3 (P3) were also included in the figure for comparison. Proteinase 3 and hCG were intact after short term storage at +4°C. However, N-elastase showed several degradation products indicating self-cleaving activity. In panel B three additional enzymes, the human mast cell chymase (HC), human thrombin (Th) and human granzyme B (GB) were included as they were used as reference proteases in the chromogenic and recombinant substrate assays.