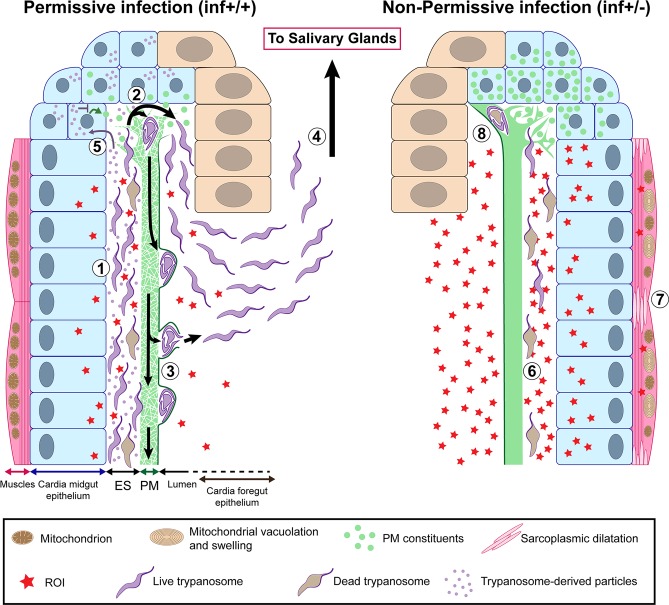
Fig 9. Model illustrating permissive (inf+/+) and non-permissive (inf+/-) infection phenotypes in tsetse’s cardia.
African trypanosomes must pass through the tsetse vector in order to complete their lifecycle and infect a new vertebrate host. Following successful infection of tsetse’s midgut, Trypanosoma brucei parasites either remain trapped indefinitely in this environment (non-permissive infection, inf +/-) or migrate to the fly’s SG where they subsequently transmit to a vertebrate host (permissive infection, inf +/+). For a inf+/+ infection to occur, trypanosomes must successfully circumvent several immunological barriers, including the cardia-synthesized PM. In this situation, parasites that have accumulated in the ES (1) of the cardia traverse the structurally compromised PM at its site of synthesis (where the matrix is most diffuse and fragile) (2) (Figs 4 and S5). The cyst-like bodies of parasites are observed within layers of the PM (S6 Fig), at which point they may force their way out by breaking through the structure’s electron-dense layer (S7 Fig). Otherwise they may stay enclosed within and move along the gut with the continuously generated PM (3) [33]. Parasites that have successfully translocated to the cardia lumen then migrate to the foregut and salivary glands (4). In an effort to facilitate their passage of the PM, trypanosomes may interfere with PM synthesis by secreting modulatory molecules (Figs 3D and 4B) that are taken up by PM synthesizing cells (5). These molecules may subsequently inhibit expression of genes that encode proteinaceous components (Pro1, Pro3, etc) of the matrix (Fig 3B) and trypanocidal reactive oxygen intermediates (ROIs). In non-permissive infections, a relatively small number of parasites reach tsetse’s cardia (Figs 2A, 2B and S2), but they appear damaged or dead (6) (Fig 8A and 8B). The inability for the parasite to sustain in the inf +/- cardia environment is likely caused by a relatively high concentration of ROIs in this environment (Fig 8C). ROI-mediated regulation of the parasite population comes with collateral damage to cardia tissues (Fig 6), especially the muscles lining the outer border of the organ, which present sarcoplasmic dilatation and mitochondrial vacuolation and swelling (7) (Figs 7 and S9). In the inf +/- cardia, PM synthesis is not affected (Fig 3B), probably due to the absence of trypanosome-derived molecules interfering with the PM production (Fig 3D). Ability to restrict parasites in the cardia prohibits the trypanosomes from translocating to the cardia lumen for subsequent transmission (8).