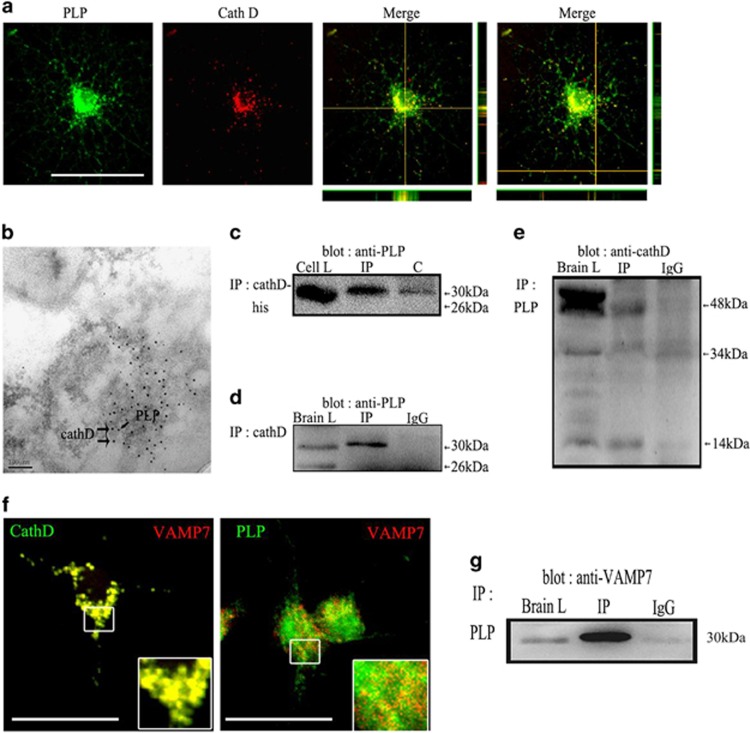
Figure 8.
CathD may regulate the transport of PLP from LEs/Ls to the plasma membrane by binding to VAMP7 and PLP. (a) Confocal imaging experiments revealed that CathD was colocalized with PLP in the perinuclear regions and processes of OPCs. (b) Immunoelectron microscopy results of the P20 C57BL/6 mouse brain. Gold particles marked CathD (larger, 15 nm particles) and PLP (smaller, 5 nm particles) colocalized in large vesicles inside LEs/Ls. (c) Co-expressed CathD and myc-tagged PLP in Hek293 cells of an in vitro pull-down assay. Myc-PLP was recovered from the immunoprecipitation of CathD. (d) Under non-denaturing conditions, antibodies against CathD precipitated PLP isoforms in the immune complexes. (e) In the reciprocal co-immunoprecipitation experiments using a goat anti-PLP antibody, only the intermediate 48-kDa and the mature light-chain 14 kDa of CathD were found to obviously co-precipitate with the PLP in the immune complex, whereas 52- kDa pro-CathD and 34-kDa mature heavy chains did not co-precipitate with the PLP in the immune complex. (f) Confocal imaging experiments revealed that PLP/VAMP7 and CathD/VAMP7 colocalized to a high degree. (g) VAMP7 was found to significantly co-precipitate with the PLP in the immune complex. Scale bar, 20 μm. CathD, cathepsin D; LE/L, late endosome/lysosome; OPC, oligodendrocyte precursor cell; PLP, proteolipid protein.