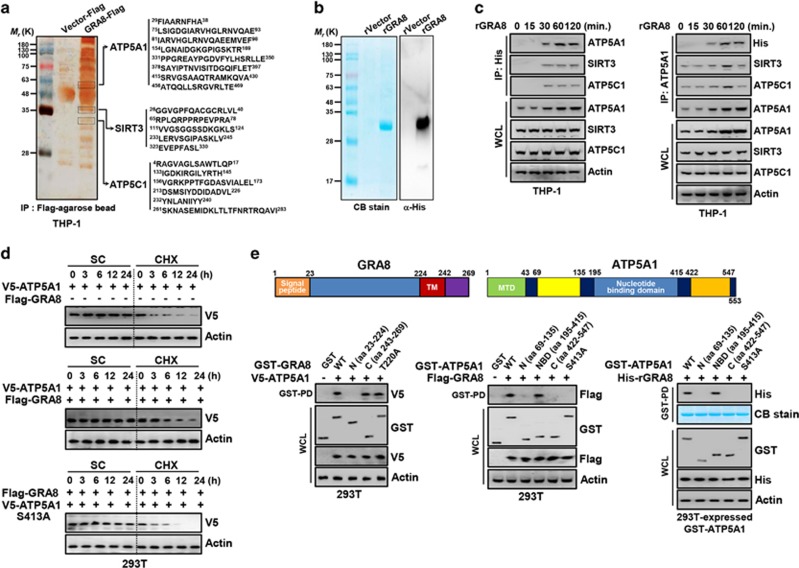
Figure 1.
GRA8 interacts with ATP5A1 and SIRT3. (a) Identification of ATP5A1, SIRT3 and ATP5C1 by mass spectrometry analysis in THP-1 cell lysates expressed with GRA8 or vector. (b) Bacterially purified 6xHis-GRA8 were analyzed by Coomassie blue staining (left) or immunoblotting (IB) with αHis (right). (c) THP-1 cells were stimulated with rGRA8 (5 μg ml−1) for the indicated times, followed by immunoprecipitation (IP) with αHis-agarose bead or αATP5A1 and IB with αATP5A1, αSIRT3, αATP5C1, αHis and αActin. (d) GRA8-mediated increases of ATP5A1 stability. At 24 h after transfection with V5-ATP5A1and/or Flag-GRA8, 293T cells were treated with solvent control (SC) or cyclohexamide (CHX, 1 μg ml−1) for indicated times and cell lysates were used for IB with αV5 and αActin. (e) Binding mapping. Schematic diagrams of the structures of GRA8 and ATP5A1 (upper). At 48 h after transfection with mammalian glutathione S-transferase (GST) or GST-GRA8 and truncated mutant constructs together with V5-ATP5A1, or GST-ATP5A1 constructs together with Flag or His-GRA8, 293T cells were used for GST pull down, followed by IB with αV5, αFlag or αHis. Whole cell lysates (WCLs) were used for IB with αGST, αV5, αFlag, αHis or αActin. The data are representative of four independent experiments with similar results (a–e).