Fig. 1.
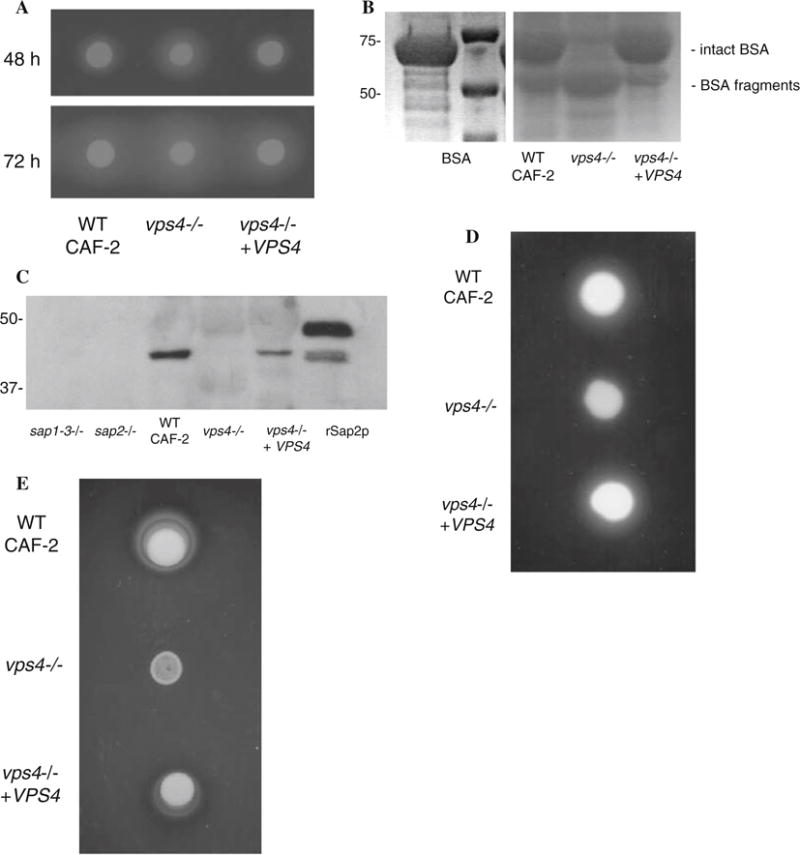
C. albicans vps4Δ mutants secrete increased extracellular protease and phospholipase activity. (a) Sap secretion by C. albicans was assayed using a plate assay for BSA degradation. Overnight cultures were spotted on BSA plates. Plates were visualized at 24 h (not shown), 48 h, and 72 h intervals. The zone of proteolysis indicates the relative amount of extracellular protease secretion. (b) Cell-free supernatants were obtained from stationary phase cells incubated in liquid BSA media, and 20 ll of each sample were analyzed by reducing SDS-polyacrylamide gel electrophoresis and Coo-massie blue staining. C. albicans vps4Δ null mutants incubated in BSA at 30°C for 14 h degraded much more extracellular BSA than wild-type strains. The smaller sized bands in this protein gel represent partially degraded BSA. This experiment indicates that the vps4Δ mutant degrades BSA to a greater extent than the control or reintegrant strains at the same time point. (c) Native Sap2p is not detected in culture supernatants of Ca vps4Δ mutants. Culture supernatants from the indicated time points were analyzed by reducing SDS-PAGE and Western blotting using polyclonal antibodies to Sap2p. rSap2p indicates purified recombinant Sap2p (from M. Monod) used as a positive control. A sap1–3Δ and a sap2Δ null mutant are used as negative controls in this experiment. (d) The vps4Δ mutant secretes reduced extracellular phospholipase activity. Overnight cultures were spotted onto egg-yolk agar plates and incubated at 37°C. The relative amount of extracellular phospholipase activity is indicated by the halo surrounding the fungal colony. (e) The vps4Δ mutant grows poorly on Tween 80 agar. Overnight cultures were spotted onto YNB–Tween 80 plates incubated at 37°C in order to assay for lipolytic activity. Unexpectedly, the vps4Δ mutant grew poorly, in comparison to the control and reintegrant strains
