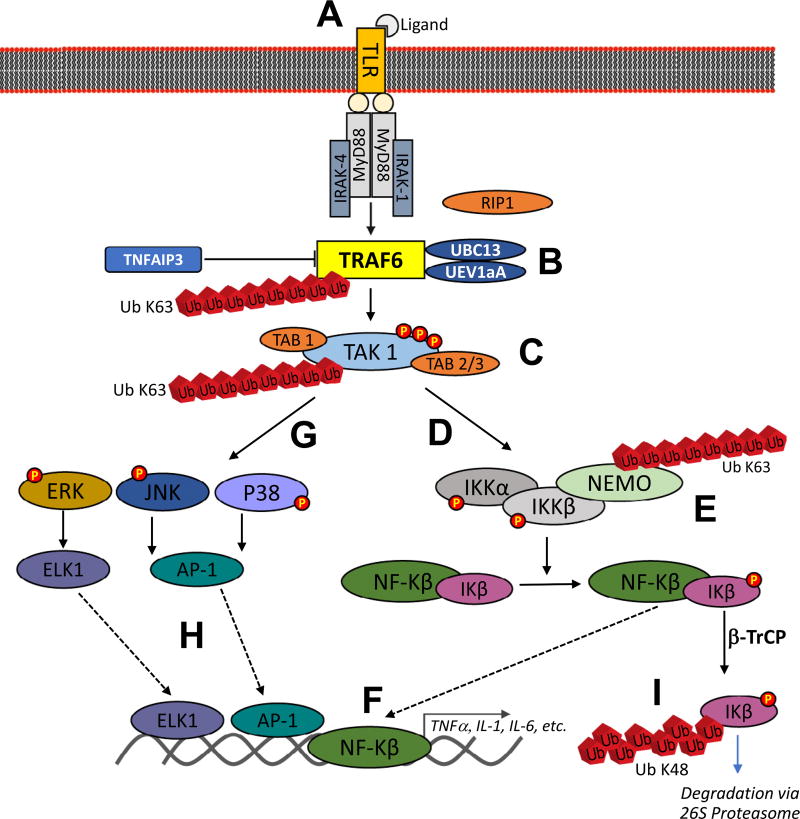
Fig 2.
Summary of TRAF6’s role in TLR-mediated activation of ERK, JNK, P38, and nuclear factor-κB. TRAF6 catalyzes K-63 polyubiquitination of TAK-1 with the help of Ubc13 and Uev1a. TAK-1 forms heterodimers with TAB1 and TAB2 or TAB3 for a strong interaction with polyubiquitin chains, phosphorylation and complete activation of TAK-1. The activated TAK-1 complex further phosphorylates and activates mitogen-activated protein kinases (MAPKs) [p38, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK)] and IκB kinase (IKK)–NF-κB pathways. Original artwork based on data summarized in Ajibade et al.34; TNFAIP3 inhibition of TRAF6 by restricting its ubiquitylation based on results published by Gui et al.35 TLR, Toll-like receptor; TRAF, tumor necrosis factor receptor-associated factor.