Abstract
Objective
Fetuin-A is a negative acute phase protein reactant that acts as a mediator for lipotoxicity, leading to insulin resistance. Intravascular inflammation and insulin resistance have been implicated in the mechanisms of disease responsible for preeclampsia (PE). Maternal plasma concentrations of fetuin-A at the time of diagnosis of preterm PE are lower than in control patients with a normal pregnancy outcome. However, it is unknown if the changes in maternal plasma fetuin-A concentrations precede the clinical diagnosis of the disease. We conducted a longitudinal study to determine whether patients who subsequently developed PE had a different profile of maternal plasma concentrations of fetuin-A as a function of gestational age (GA) than those with uncomplicated pregnancies.
Methods
A longitudinal case-control study was performed and included 200 singleton pregnancies in the following groups: 1) patients with uncomplicated pregnancies who delivered appropriate for gestational age (AGA) neonates (n = 160); and 2) patients who subsequently developed PE (n = 40). Longitudinal samples were collected at each prenatal visit and scheduled at 4-week intervals from the first or early second trimester until delivery. Plasma fetuin-A concentrations were determined by ELISA. Analysis was performed using mixed-effects models.
Results
The profiles of maternal plasma concentrations of fetuin-A differ between PE and uncomplicated pregnancies. Forward analysis indicated that the rate of increase of plasma fetuin-A concentration in patients who subsequently developed PE was lower at the beginning of pregnancy (p=0.001), yet increased faster in mid-pregnancy (p=0.0017) and reached the same concentration level as controls by 26 weeks. The rate of decrease was higher towards the end of pregnancy in patients with PE than in uncomplicated pregnancies (p=0.002). The mean maternal plasma fetuin-A concentration was significantly lower in patients with preterm PE at the time of clinical diagnosis than in women with uncomplicated pregnancies (p <0.05). In contrast, there were no significant differences in maternal plasma fetuin-A concentration in patients who developed PE at term.
Conclusions
(1) The profile of maternal plasma concentrations of fetuin-A over time (GA) in patients who develop PE is different from that of normal pregnant women; (2) the rate of change of maternal plasma concentrations of fetuin-A is positive (increases over time) in the midtrimester of normal pregnancy, and negative (decreases over time) in patients who subsequently develop PE; (3) at the time of diagnosis, the maternal plasma fetuin-A concentration is lower in patients with preterm PE than in those with a normal pregnancy outcome; however, such differences were not demonstrable in patients with term PE.
Keywords: α2-Heremans-Schmid glycoprotein, hypertensive disorders in pregnancy, insulin resistance, intravascular inflammation, negative acute phase protein reactant
Introduction
Preeclampsia (PE), one of the “great obstetrical syndromes” [1–4], is a leading cause of maternal [5–19] and neonatal morbidity [20–27]. Several mechanisms of disease have been implicated in the genesis of the syndrome, including: (1) failure of physiologic transformation of the spiral arteries [28–34]; (2) an anti-angiogenic state [35–69]; (3) systemic intravascular inflammation [70–77]; (4) endothelial dysfunction [78–84]; (5) oxidative stress [84–89]; (6) endoplasmic reticulum stress [88,90]; (7) platelet [91–95] and thrombin activation [96–103]; (8) anti-angiotensin-II antibodies [104–113]; and (9) insulin resistance [114–137].
Fetuin-A, a negative acute phase protein reactant (plasma protein whose concentration decreases during an inflammatory state [138–142]), has been implicated in the pathophysiology of PE [143–148]. This fetal protein was first described by Kai Pedersen [149]. It has a very high concentration in the neonatal calf, is mainly produced by the liver and decreases with time; therefore, he proposed that the protein be called “Fetuin” (from the Latin “Foetus”) [149]. In 1990, homology between human α2-Heremans-Schmid glycoprotein (AHSG) and fetuin in cattle was demonstrated [150]. Fetuin-A can inhibit LPS- or IFN-γ-induced high mobility group box-1 proteins (HMGB-1) released in macrophages [142,151]. Moreover, fetuin-A may influence the resolution of inflammation by acting as a bacterial opsonin, thereby facilitating macrophage-mediated ingestion and the elimination of apoptotic neutrophils [152–154]. The administration of fetuin-A can protect against death during the course of endotoxemia and experimental sepsis induced by cecal puncture ligation [151]. Fetuin-A is also a hepatokine with metabolic effects [155–160]. Specifically, this protein has been identified as a mediator of lipotoxicity through the toll-like receptor-4 pathway, and can lead to insulin resistance [161].
We have reported that the maternal plasma concentrations of fetuin-A are significantly lower in women with preterm PE at the time of diagnosis than in normal pregnant women [162]. Moreover, such concentrations are correlated with the concentrations of other negative acute phase reactants, such as albumin and transferrin, in patients with PE. There is no information on whether the changes in fetuin-A occur prior to the development of PE. This longitudinal study was performed to address this question.
Materials and methods
Study design and participants
This retrospective, longitudinal, nested case-control study included 200 singleton pregnancies in the following groups: 1) women with uncomplicated pregnancies who delivered an appropriate for gestational age (AGA) neonate (controls; n=160); and 2) patients who had PE (n=40). Multiple gestations, pregnancies with fetal congenital anomalies and pregnancies complicated by chronic hypertension, diabetes mellitus, and/or renal disease were excluded.
Plasma samples were obtained serially during pregnancy. All patients had a minimum of three samples during pregnancy (range: three to seven samples). Plasma samples were selected once from each patient at the following seven intervals: 6–14.9; 15–19.9; 20–24.9; 25–27.9; 28–31.9; 32–36.9; 37–41.1 weeks of gestation. The earliest sample for each interval was used if multiple samples had been collected in a particular window. Samples collected after the clinical diagnosis of PE were excluded.
Clinical definition
PE was diagnosed in the presence of systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg on at least two occasions, 4 h to 1 week apart, and proteinuria >300 mg in 24-h urine collection or one dipstick with >2+ urine protein [163, 164]. Pregnant women were considered “normal” if they had no medical, obstetrical or surgical complications, and delivered a normal term (>37 weeks) infant whose birthweight was AGA (10th–90th percentile) [165,166].
The collection and utilization of the samples was approved by both the Human Investigation Committee of the Sótero del Rió Hospital, Santiago, Chile (a major affiliate for the Catholic University of Santiago) and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (National Institutes of Health, Department of Health and Human Services). Many of these samples were used in previous studies.
Sample collection and fetuin-A immunoassays
Blood samples were collected into tubes containing EDTA, then centrifuged for 10 min at 4°C and stored at −70°C. Laboratory personnel were blinded to clinical diagnosis. Maternal plasma concentrations of fetuin-A were determined using sensitive and specific immunoassays (BioVender LLC, Candler, NC). The immunoassay was performed in duplicate and utilized a sandwich enzyme-based technique and had been validated for plasma determinations of the analytes. The inter- and intra-assay coefficients of variation were 4.7% and 2.9%, respectively. The sensitivity of the assays was 4.1 ng/mL.
Statistical analysis
Cross-sectional analysis of demographic and clinical characteristic data
The Kolmogorov-Smirnov test was used to assess the distribution of the data. Since the data were not normally distributed, we used the Kruskal-Wallis test for comparisons among groups and the Mann-Whitney U test for comparisons between groups for continuous variables. Chi-square or Fisher’s exact tests were used for comparisons of categorical variables. Statistical analysis was performed using SPSS 19 (IBM Corp, Armonk, NY) and SAS 9.3 (Cary, NC). A p value <0.05 was considered statistically significant.
Longitudinal analysis of plasma fetuin-A concentration
The data collected in this study contain serial measurements from each individual belonging to two groups (PE and controls). We used linear mixed effects models for analysis, which included fixed and random effects [167,168]. Using this approach, each subject has its own baseline response (fetuin-A concentration; random intercept) but is assumed to follow the same (fixed) profile over time (GA). The fixed effects included the diagnostic group (PE versus controls), polynomial terms of the GA at venipuncture up to the third degree, body mass index (BMI) (kg/m2), and the duration of sample storage (years). Parity and maternal age were tested but did not improve the model fit as determined by a likelihood ratio test. In addition, interaction terms between polynomial components of GA and the diagnostic group were also considered fixed effects. The effect of the group (difference in the mean fetuin-A concentration between women with PE and controls) was assessed at various GA (10–41 weeks) using the linear mixed effects model.
The Imer function from the Ime4 package under the R statistical environment (www.r-project.org) was used for mixed-effects model fitting. Significance of the fixed effects in the linear model was determined using the ANOVA method in the Ime4 package, which performs a likelihood ratio test between the model fit with and without the fixed effects of interest. A p value <0.05 was considered significant.
Backward longitudinal analysis
Each patient with PE was matched with up to four controls based on GA at sampling. Samples from matched controls collected after delivery of patients with PE were excluded from the analysis. The GA at the time of diagnosis of patients with PE and their matched controls was subtracted from the GA at sampling to compute the number of weeks before the diagnosis. The same mixed effects model used in the forward analysis was also applied to test for differences in the plasma concentrations of fetuin-A as a function of the time to diagnosis.
Results
Demographic and clinical characteristics of patients across the study groups
This study included a total of 1328 samples (1101, uncomplicated pregnancies; 227, patients with PE). The demographic and clinical characteristics of the study groups are displayed in Table 1. Patients who developed PE had a significantly lower median maternal age but higher median pre-pregnancy BMI and proportion of nulliparous women than those with uncomplicated pregnancies. There was no significant difference in the median GA at enrollment between patients who subsequently developed PE and those with uncomplicated pregnancies. The median GA at delivery and birthweight were lower in patients with PE than in the uncomplicated pregnancy group (p<0.001 both; Table 1). Twenty-seven percent (11/40) of patients with PE delivered a small-for-gestational-age (SGA) neonate, and 37.5% (15/40) delivered before 37 weeks of gestation.
Table 1.
Demographic and clinical characteristics of the study groups
| Uncomplicated pregnancy (n=160) | Preeclampsia (n=40) | p | |
|---|---|---|---|
| Maternal age (years) | 24 (20–30) | 20.5 (19–25) | 0.02 |
| Pre-pregnancy body mass index (BMI) (kg/m2) | 23.9 (21.6–26.8) | 25.7 (23.5–29.2) | 0.005 |
| Smoking | 23 (14.4) | 2 (5) | 0.55 |
| Nulliparity | 71 (44.4) | 26 (65) | 0.02 |
| Gestational age at enrollment (weeks) | 9.4 (8–11.4) | 10 (8.7–11.4) | 0.3 |
| Gestational age at delivery (weeks) | 40 (39.2–40.7) | 38 (34.8–39.3) | <0.001 |
| Birthweight (g) | 3415 (3190–3560) | 2980 (1990–3537) | <0.001 |
| Birthweight < 10th percentile | 0 | 11(27.5) | <0.001 |
| Delivery < 37 weeks | 0 | 15 (37.5) | <0.001 |
Data expressed as median (interquartile range) and number (percentage).
Maternal plasma concentrations of fetuin-A in preeclampsia (forward longitudinal analysis)
The average profile of maternal plasma fetuin-A concentration as a function of GA was different between women who developed PE and the control group [p<0.05 for the interaction terms between PE and polynomial components of the GA (“PE × GA,” “PE × GA2,” and “PE × GA3”) of the mixed effects model (Table 2).
Table 2.
Longitudinal analysis of the association between fetuin-A and preeclampsia after adjusting for confounding factors
| All Preeclampsia vs. Uncomplicated Pregnancy | ||||
|---|---|---|---|---|
| Estimate | Standard Error | t-value | p-value | |
| Intercept | 265.7 | 5.3 | 49.89 | <0.001 |
| (GA-41) | −4.9 | 1.2 | −4.25 | <0.001 |
| (GA-41)2 | −0.198 | 0.088 | −2.24 | 0.01 |
| (GA-41)3 | −0.0014 | 0.0019 | −0.77 | 0.22 |
| Group | −43.2 | 15.1 | −2.87 | 0.002 |
| BMI-25 | 1.63 | 0.72 | 2.27 | 0.01 |
| Storage-8.13 | −27.0 | 4.6 | −5.82 | <0.001 |
| (GA-41) × PE | −10.3 | 3.4 | −3.06 | 0.001 |
| (GA-41)2 × PE | −0.700 | 0.238 | −2.94 | 0.002 |
| (GA-41)3 × PE | −0.014 | 0.005 | −2.86 | 0.002 |
GA, gestational age; BMI, body mass index; PE, preeclampsia; (GA-41) × PE, interaction term between diagnosis and GA; (GA-41)2 × PE, interaction between diagnosis and GA2; (GA-41)3 × PE, interaction between diagnosis and GA3.
The changes in plasma concentrations of fetuin-A in patients who subsequently developed PE and those who had uncomplicated pregnancies across all GA are displayed in Figure 1. The curves in the figures represent a polynomial fit of the analyte concentration as a function of GA among uncomplicated pregnant women and those who developed PE (after adjusting for BMI and storage time).
Figure 1. Maternal plasma concentrations of fetuin-A in women with uncomplicated pregnancy (black dot) and patients who subsequently developed preeclampsia (PE) (red dot).
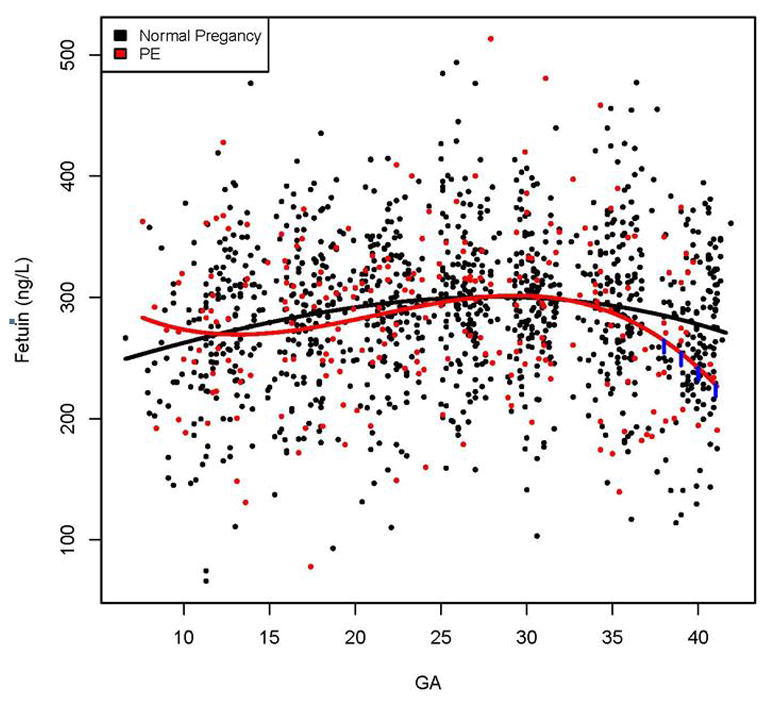
The gestational age dependence of the fetuin-A concentration in uncomplicated pregnant women (black line) and those in preeclampsia group (red line) was estimated using linear mixed-effects using a third degree polynomial function. The vertical lines on the preeclampsia curve denote statistical significance of the difference between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates [body mass index (BMI) (kg/m2) and the duration of sample storage (years)].
The rate of the increase in plasma fetuin-A concentration in patients who subsequently developed PE was lower at the beginning of pregnancy (p=0.001), yet increased faster in the midtrimester of pregnancy (p=0.0017) to reach a similar concentration as uncomplicated pregnancy by 26 weeks. Subsequently, the rate of decrease was greater towards the end of gestation in women who developed PE than in those who had uncomplicated pregnancies (p=0.002; Figure 1).
Maternal plasma fetuin-A concentrations before the diagnosis of preeclampsia
While the change over time in maternal plasma concentration of fetuin-A is different in women who subsequently developed PE from women who have a normal pregnancy outcome, an important clinical question is whether the absolute concentrations are different at any given time before diagnosis – this could have implications for the prediction of the syndrome. Therefore, we conducted a backward analysis to address this specific question. The mean maternal plasma fetuin-A concentration was significantly lower in patients with preterm PE at the time of diagnosis than in those with uncomplicated pregnancies (p<0.05; Table 3), but not before. However, there was no significant difference in the mean maternal plasma fetuin-A concentration before or at the time of clinical diagnosis between patients with term PE and those with uncomplicated pregnancies (Table 4).
Table 3.
The difference in plasma fetuin-A concentration and the statistical differences (p value) according to weeks before clinical diagnosis between patients with preterm preeclampsia and uncomplicated pregnancies
| Weeks before clinical diagnosis | Difference in plasma fetuin-A concentration between patients with preterm preeclampsia and uncomplicated pregnancies | P value |
|---|---|---|
| 0 | −31.46 | 0.03 |
| −1 | −20.10 | 0.14 |
| −2 | −11.00 | 0.40 |
| −3 | −3.98 | 0.76 |
| −4 | 1.12 | 0.93 |
| −5 | 4.49 | 0.74 |
| −6 | 6.29 | 0.65 |
| −7 | 6.69 | 0.63 |
| −8 | 5.88 | 0.66 |
| −9 | 4.02 | 0.76 |
| −10 | 1.28 | 0.92 |
| −11 | −2.15 | 0.87 |
| −12 | −6.11 | 0.64 |
| −13 | −10.43 | 0.42 |
| −14 | −14.93 | 0.26 |
| −15 | −19.43 | 0.15 |
Table 4.
The difference in plasma fetuin-A concentration and the statistical differences (p value) according to weeks before clinical diagnosis between patients with term preeclampsia and uncomplicated pregnancies
| Weeks before clinical diagnosis | Difference in plasma fetuin-A concentration between patients with term preeclampsia and uncomplicated pregnancies | P value |
|---|---|---|
| 0 | −10.84 | 0.42 |
| −1 | −4.97 | 0.69 |
| −2 | −0.20 | 0.99 |
| −3 | 3.56 | 0.76 |
| −4 | 6.40 | 0.59 |
| −5 | 8.40 | 0.48 |
| −6 | 9.66 | 0.42 |
| −7 | 10.26 | 0.40 |
| −8 | 10.29 | 0.39 |
| −9 | 9.84 | 0.41 |
| −10 | 8.99 | 0.45 |
| −11 | 7.84 | 0.51 |
| −12 | 6.47 | 0.58 |
| −13 | 4.97 | 0.67 |
| −14 | 3.42 | 0.77 |
| −15 | 1.93 | 0.87 |
Discussion
Principal findings
(1) This is the first longitudinal study reporting a change in plasma fetuin-A concentrations in patients who subsequently developed PE; (2) the rate of change of maternal plasma concentrations of fetuin-A is positive (increases over time) in the midtrimester of normal pregnancy, and negative (decreases over time) in patients who subsequently developed PE; and (3) the mean maternal plasma fetuin-A concentration was significantly lower in patients with preterm PE than in those with uncomplicated pregnancies at the time of clinical diagnosis.
Maternal plasma fetuin-A concentration and preeclampsia
Previous studies examining serum concentrations of fetuin-A in PE yield conflicting results. Gomez et al. [147] and Park et al. [146] reported that the serum concentration of this protein was increased in patients with PE, while Movalec et al. [143,144] reported that it was lower in women with PE than in those with an uncomplicated pregnancy.
Our findings demonstrate that the profile (concentration over time) of maternal plasma fetuin-A among patients who subsequently developed PE throughout pregnancy differs from that of uncomplicated pregnancies. The mean fetuin-A maternal plasma concentration was lower in preterm PE at the time of diagnosis: these observations are consistent with a previous cross-sectional study at the time of diagnosis of PE performed in two different populations (Hispanics and African-Americans) [162] and with those of Molvarec et al. [143,144] who reported that median serum fetuin-A concentrations were lower in women with PE and patients with HELLP syndrome compared to women with uncomplicated pregnancies. Moreover, fetuin-A concentrations showed significant inverse correlations with serum C-reactive protein concentration [143] while having a significant positive correlation with plasma albumin and transferrin [162] in women with PE, supporting the role of fetuin-A as an acute negative phase protein reactant.
Fetuin-A: a negative acute-phase reactant protein
A likely explanation for the lower concentration of fetuin-A in PE is that this protein is a negative acute-phase reactant. PE is characterized by intravascular inflammation [70–77], demonstrated with flow cytometry studies [73] as well as the determination of cytokines/chemokines [169–181], complement split products [182], etc. Moreover, maternal peripheral blood mononuclear cells from patients with PE produced higher concentrations of proinflammatory cytokines than those with uncomplicated pregnancies [173,174,178,183–187].
Evidence that fetuin-A acts as a negative acute phase protein reactant [139–141] includes: (1) in patients with bacterial infection, serum fetuin-A concentrations decreased, and had a positive correlation with acute negative positive phase protein reactants (e.g. alpha anti-trypsin, orosomucoid and haptoglobin) and a positive correlation with acute negative phase reactants (e.g. albumin and transferrin) [139]; (2) the administration of recombinant human interleukin-6 and interleukin-1β decreases the synthesis of fetuin in human hepatoma HepG2 cells [140]; and (3) circulating fetuin-A concentrations decrease at approximately 24–48 h, returning toward the base-line approximately 72 h after onset of endotoxemia or sepsis in a mouse model of lethal systemic inflammation (induced by endotoxemia or cecal puncture ligation) [151].
In a previous study [162], we reported that patients with two pregnancy complications (PE and acute pyelonephritis) in which there is intravascular inflammation [73,188] have lower concentrations of fetuin-A than normal pregnant women. Moreover, we demonstrated that there was a significant correlation between the concentrations of fetuin-A and two other acute negative-phase reactants – albumin and transferrin – particularly among women with PE [162]. One potential explanation for the lack of demonstrable difference in the concentration of fetuin-A between patients with PE at term and normal pregnancy may be that intravascular inflammation is less severe in term than in preterm PE [189,190].
Another possible mechanism for the decreased plasma fetuin-A concentrations in PE is explained by the protective role of vascular calcification of fetuin-A [191–196]. Fetuin-A knock-out mice developed severe calcification in multiple organs [192], and the administration of fetuin-A to cultured bovine vascular smooth muscle cell resulted in stiffness of mineralization [193]. In humans, plasma fetuin-A concentration had an inverse correlation with the arterial stiffness determined by flow-mediated dilatation in both healthy subjects [194] and those with chronic kidney disease [195–198]. Endothelial dysfunction and increased arterial stiffness have been detected in patients with PE [199–201].
Strengths and limitations of this study
This is the first longitudinal study that evaluated the changes of the maternal plasma concentration of fetuin-A in patients with PE from the first trimester of pregnancy. Due to substantial overlapping in absolute concentrations, we believe it is unlikely that fetuin-A concentrations could be a useful biomarker for the prediction of PE.
Conclusion
The profile of maternal plasma fetuin-A concentration (over time) differs between patients who subsequently developed PE and those with uncomplicated pregnancies. The most likely explanation for the lower concentration of fetuin-A in maternal plasma in patients with preterm PE at the time of diagnosis is that this protein is a negative acute phase reactant, and therefore, its concentration may reflect the presence of intravascular inflammation.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
Footnotes
Presented as an oral presentation at the World Congress of Perinatal Medicine, June 19–22, 2013, Moscow, Russia
Disclosure: The authors report no conflicts of interest.
References
- 1.Romero R, Lockwood C, Oyarzun E, et al. Toxemia: new concepts in an old disease. Semin Perinatol. 1988;12:302–23. [PubMed] [Google Scholar]
- 2.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 3.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–5. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 4.Romero R. Prenatal medicine: the child is the father of the man 1996. J Matern Fetal Neonatal Med. 2009;22:636–9. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 5.Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307–12. doi: 10.1016/s0002-9378(00)70216-x. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–9. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 8.von Dadelszen P, Menzies J, Magee LA. The complications of hypertension in pregnancy. Minerva Med. 2005;96:287–302. [PubMed] [Google Scholar]
- 9.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 10.Sibai BM. Hypertensive disorders of pregnancy: the United States perspective. Curr Opin Obstet Gynecol. 2008;20:102–6. doi: 10.1097/GCO.0b013e3282f73380. [DOI] [PubMed] [Google Scholar]
- 11.Clark SL, Belfort MA, Dildy GA, et al. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199:36e1–5. doi: 10.1016/j.ajog.2008.03.007. discussion 91–2. e7–11. [DOI] [PubMed] [Google Scholar]
- 12.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 13.Habli M, Eftekhari N, Wiebracht E, et al. Long-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am J Obstet Gynecol. 2009;201:385.e381–5. doi: 10.1016/j.ajog.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Berg CJ, Mackay AP, Qin C, et al. Overview of maternal morbidity during hospitalization for labor and delivery in the United States:1993–1997 and 2001–2005. Obstet Gynecol. 2009;113:1075–81. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 15.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of preeclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Cruz MO, Gao W, Hibbard JU. Obstetrical and perinatal outcomes among women with gestational hypertension, mild preeclampsia, and mild chronic hypertension. Am J Obstet Gynecol. 2011;205:260.e261–9. doi: 10.1016/j.ajog.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Thornton C, Dahlen H, Korda A, et al. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. Am J Obstet Gynecol. 2013;208:476.e471–5. doi: 10.1016/j.ajog.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 18.Vrachnis N, Vlachadis N, Iliodromiti Z. The incidence of preeclampsia and eclampsia in Australia: 2000 through 2008. Am J Obstet Gynecol. 2014;210:173–4. doi: 10.1016/j.ajog.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Nakimuli A, Chazara O, Byamugisha J, et al. Pregnancy, parturition and preeclampsia in women of African ancestry. Am J Obstet Gynecol. 2014;210:510–20. doi: 10.1016/j.ajog.2013.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witlin AG, Saade GR, Mattar F, et al. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2000;182:607–11. doi: 10.1067/mob.2000.104224. [DOI] [PubMed] [Google Scholar]
- 21.Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–90. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 22.Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol. 2006;30:16–19. doi: 10.1053/j.semperi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Ganzevoort W, Rep A, de Vries JI, et al. Prediction of maternal complications and adverse infant outcome at admission for temporizing management of early-onset severe hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2006;195:495–503. doi: 10.1016/j.ajog.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Habli M, Levine RJ, Qian C, et al. Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. Am J Obstet Gynecol. 2007;197:406.e1–7. doi: 10.1016/j.ajog.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 25.Chappell LC, Enye S, Seed P, et al. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension. 2008;51:1002–9. doi: 10.1161/HYPERTENSIONAHA.107.107565. [DOI] [PubMed] [Google Scholar]
- 26.Langenveld J, Ravelli AC, van Kaam AH, et al. Neonatal outcome of pregnancies complicated by hypertensive disorders between 34 and 37 weeks of gestation: a 7 year retrospective analysis of a national registry. Am J Obstet Gynecol. 2011;205:540.e1–7. doi: 10.1016/j.ajog.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Belghiti J, Kayem G, Tsatsaris V, et al. Benefits and risks of expectant management of severe preeclampsia at less than 26 weeks gestation: the impact of gestational age and severe fetal growth restriction. Am J Obstet Gynecol. 2011;205:465.e1–6. doi: 10.1016/j.ajog.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 28.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–79. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 29.Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972;79:794–9. doi: 10.1111/j.1471-0528.1972.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 30.Khong TY, De Wolf F, Robertson WB, et al. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 31.Pijnenborg R, Brosens I. Deep trophoblast invasion and spiral artery remodeling. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders. Cambridge: Cambridge University Press; 2010. pp. 97–108. [Google Scholar]
- 32.Romero R, Kusanovic JP, Kim CJ. Disorders of the placental bed in the genesis of the great obstetrical syndromes. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders. Cambridge: Cambridge University Press; 2010. pp. 271–89. [Google Scholar]
- 33.Brosens I, Pijnenborg R, Vercruysse L, et al. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Kusanovic JP, Chaiworapongsa T, et al. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011;25:313–27. doi: 10.1016/j.bpobgyn.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, McMaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–23. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 38.Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 39.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–7. doi: 10.1016/j.ajog.2004.03.043. discussion 1547–50. [DOI] [PubMed] [Google Scholar]
- 40.Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302. doi: 10.1111/j.1471-0528.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 41.Nagamatsu T, Fujii T, Kusumi M, et al. Cytotrophoblasts upregulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–45. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 42.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad S, Ahmed A. Antiangiogenic effect of soluble vascular endothelial growth factor receptor-1 in placental angiogenesis. Endothelium. 2005;12:89–95. doi: 10.1080/10623320590933888. [DOI] [PubMed] [Google Scholar]
- 44.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 45.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 46.Vatten LJ, Eskild A, Nilsen TI, et al. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239.e.1–6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 47.Chaiworapongsa T, Romero R, Gotsch F, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stepan H. Angiogenic factors and pre-eclampsia: an early marker is needed. Clin Sci (Lond) 2009;116:231–2. doi: 10.1042/CS20080598. [DOI] [PubMed] [Google Scholar]
- 52.Chaiworapongsa T, Romero R, Kusanovic JP, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–62. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silasi M, Cohen B, Karumanchi SA, et al. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37:239–53. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in earlyonset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–52. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25:498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagmann H, Thadhani R, Benzing T, et al. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem. 2012;58:837–45. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 58.Weed S, Bastek JA, Anton L, et al. Examining the correlation between placental and serum placenta growth factor in preeclampsia. Am J Obstet Gynecol. 2012;207:140.e1–6. doi: 10.1016/j.ajog.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Weissgerber TL, Roberts JM, Jeyabalan A, et al. Haptoglobin phenotype, angiogenic factors, and preeclampsia risk. Am J Obstet Gynecol. 2012;206:358.e10–18. doi: 10.1016/j.ajog.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rana S, Cerdeira AS, Wenger J, et al. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One. 2012;7:e48259. doi: 10.1371/journal.pone.0048259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McElrath TF, Lim KH, Pare E, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207:407.e1–7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128:2121–31. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 63.Goel A, Rana S. Angiogenic factors in preeclampsia: potential for diagnosis and treatment. Curr Opin Nephrol Hypertens. 2013;22:643–50. doi: 10.1097/MNH.0b013e328365ad98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verlohren S, Herraiz I, Lapaire O, et al. New gestational phasespecific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–52. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- 65.Schaarschmidt W, Rana S, Stepan H. The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP syndrome. J Perinat Med. 2013;41:511–16. doi: 10.1515/jpm-2012-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208:287.e1–15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore Simas TA, Crawford SL, Bathgate S, et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J Matern Fetal Neonatal Med. 2014;27:1038–48. doi: 10.3109/14767058.2013.847415. [DOI] [PubMed] [Google Scholar]
- 68.Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132–44. doi: 10.3109/14767058.2013.806905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verlohren S, Herraiz I, Lapaire O, et al. New gestational phasespecific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–52. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- 70.Sacks GP, Studena K, Sargent K, et al. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 71.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessivematernal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 72.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 73.Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 74.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response–a review. Placenta. 2003;24:S21–27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 75.Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol. 2004;24:565–70. doi: 10.1016/s0270-9295(04)00127-5. [DOI] [PubMed] [Google Scholar]
- 76.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(Suppl A):S73–77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Redman CW, Tannetta DS, Dragovic RA, et al. Review: does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33(Suppl):S48–54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Roberts JM, Taylor RN, Musci TJ, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 79.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–8. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 80.Friedman SA, Schiff E, Emeis JJ, et al. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol. 1995;172:202–3. doi: 10.1016/0002-9378(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 81.Lyall F, Greer IA. The vascular endothelium in normal pregnancy and pre-eclampsia. Rev Reprod. 1996;1:107–16. doi: 10.1530/ror.0.0010107. [DOI] [PubMed] [Google Scholar]
- 82.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 83.Taylor RN, de Groot CJ, Cho YK, et al. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 84.Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30(Suppl A):S55–65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–35. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 86.Myatt L, Kossenjans W, Sahay R, et al. Oxidative stress causes vascular dysfunction in the placenta. J Matern Fetal Med. 2000;9:79–82. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<79::AID-MFM16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 87.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregn. 2002;21:205–23. doi: 10.1081/PRG-120015848. [DOI] [PubMed] [Google Scholar]
- 88.Burton GJ, Yung HW, Cindrova-Davies T, et al. Placentalendoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou X, Zhang GY, Wang J, et al. A novel bridge between oxidative stress and immunity: the interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am J Obstet Gynecol. 2012;206:447.e7–16. doi: 10.1016/j.ajog.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Lian IA, Loset M, Mundal SB, et al. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta. 2011;32:823–9. doi: 10.1016/j.placenta.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rakoczi I, Tallian F, Bagdany S, et al. Platelet life-span in normal pregnancy and pre-eclampsia as determined by a non-radioisotope technique. Thromb Res. 1979;15:553–6. doi: 10.1016/0049-3848(79)90161-0. [DOI] [PubMed] [Google Scholar]
- 92.Socol ML, Weiner CP, Louis G, et al. Platelet activation in preeclampsia. Am J Obstet Gynecol. 1985;151:494–7. doi: 10.1016/0002-9378(85)90276-5. [DOI] [PubMed] [Google Scholar]
- 93.Csaicsich P, Deutinger J, Tatra G. Platelet specific proteins (betathromboglobulin and platelet factor 4) in normal pregnancy and in pregnancy complicated by preeclampsia. Arch Gynecol Obstet. 1989;244:91–5. doi: 10.1007/BF00931379. [DOI] [PubMed] [Google Scholar]
- 94.Fitzgerald DJ, Rocki W, Murray R, et al. Thromboxane A2 synthesis in pregnancy-induced hypertension. Lancet. 1990;335:751–4. doi: 10.1016/0140-6736(90)90869-7. [DOI] [PubMed] [Google Scholar]
- 95.Ahmed Y, van Iddekinge B, Paul C, et al. Retrospective analysis of platelet numbers and volumes in normal pregnancy and in preeclampsia. Br J Obstet Gynaecol. 1993;100:216–20. doi: 10.1111/j.1471-0528.1993.tb15233.x. [DOI] [PubMed] [Google Scholar]
- 96.Cunningham FG, Pritchard JA. Hematologic considerations of pregnancy-induced hypertension. Semin Perinatol. 1978;2:29–38. [PubMed] [Google Scholar]
- 97.Weenink GH, Treffers PE, Vijn P, et al. Antithrombin III levels in preeclampsia correlate with maternal and fetal morbidity. Am J Obstet Gynecol. 1984;148:1092–7. doi: 10.1016/0002-9378(84)90634-3. [DOI] [PubMed] [Google Scholar]
- 98.de Boer K, ten Cate JW, Sturk A, et al. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989;160:95–100. doi: 10.1016/0002-9378(89)90096-3. [DOI] [PubMed] [Google Scholar]
- 99.Cadroy Y, Grandjean H, Pichon J, et al. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br J Obstet Gynaecol. 1993;100:416–20. doi: 10.1111/j.1471-0528.1993.tb15264.x. [DOI] [PubMed] [Google Scholar]
- 100.Chaiworapongsa T, Yoshimatsu J, Espinoza J, et al. Evidence of in vivo generation of thrombin in patients with small-forgestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11:362–7. doi: 10.1080/jmf.11.6.362.367. [DOI] [PubMed] [Google Scholar]
- 101.Dekker G. Prothrombotic mechanisms in preeclampsia. Thromb Res. 2005;115:17–21. [PubMed] [Google Scholar]
- 102.Erez O, Romero R, Kim SS, et al. Over-expression of the thrombin receptor (PAR-1) in the placenta in preeclampsia: a mechanism for the intersection of coagulation and inflammation. J Matern Fetal Neonatal Med. 2008;21:345–55. doi: 10.1080/14767050802034859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erez O, Romero R, Hoppensteadt D, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Matern Fetal Neonatal Med. 2008;21:855–69. doi: 10.1080/14767050802361872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallukat G, Neichel D, Nissen E, et al. Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Can J Physiol Pharmacol. 2003;81:79–83. doi: 10.1139/y02-160. [DOI] [PubMed] [Google Scholar]
- 106.LaMarca B. Progress toward identifying potential markers for preeclampsia: role of agonistic autoantibody to the angiotensin II type I receptor. Hypertension. 2010;55:236–7. doi: 10.1161/HYPERTENSIONAHA.109.141465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parrish MR, Murphy SR, Rutland S, et al. The effect of immunefactors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010;23:911–16. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siddiqui AH, Irani RA, Blackwell SC, et al. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia:correlation with disease severity. Hypertension. 2010;55:386–93. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Irani RA, Zhang Y, Zhou CC, et al. Autoantibody-mediatedangiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension. 2010;55:1246–53. doi: 10.1161/HYPERTENSIONAHA.110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.LaMarca B, Wallace K, Granger J. Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia. Curr Opin Pharmacol. 2011;11:175–9. doi: 10.1016/j.coph.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LaMarca B, Parrish MR, Wallace K. Agonistic autoantibodies to the angiotensin II type I receptor cause pathophysiologic characteristics of preeclampsia. Gend Med. 2012;9:139–46. doi: 10.1016/j.genm.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 2013;113:78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herse F, LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am J Reprod Immunol. 2013;69:413–18. doi: 10.1111/aji.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garner PR, D’Alton ME, Dudley DK, et al. Preeclampsia in diabetic pregnancies. Am J Obstet Gynecol. 1990;163:505–8. doi: 10.1016/0002-9378(90)91184-e. [DOI] [PubMed] [Google Scholar]
- 115.Berkowitz GS, Roman SH, Lapinski RH, et al. Maternal characteristics, neonatal outcome, and the time of diagnosis of gestational diabetes. Am J Obstet Gynecol. 1992;167:976–82. doi: 10.1016/s0002-9378(12)80023-8. [DOI] [PubMed] [Google Scholar]
- 116.Greco P, Loverro G, Selvaggi L. Does gestational diabetes represent an obstetrical risk factor? Gynecol Obstet Invest. 1994;37:242–5. doi: 10.1159/000292569. [DOI] [PubMed] [Google Scholar]
- 117.Kaaja R, Tikkanen MJ, Viinikka L, et al. Serum lipoproteins, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstet Gynecol. 1995;85:353–6. doi: 10.1016/0029-7844(94)00380-V. [DOI] [PubMed] [Google Scholar]
- 118.Kaaja R. Insulin resistance syndrome in preeclampsia. Semin Reprod Endocrinol. 1998;16:41–6. doi: 10.1055/s-2007-1016251. [DOI] [PubMed] [Google Scholar]
- 119.Joffe GM, Esterlitz JR, Levine RJ, et al. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1998;179:1032–7. doi: 10.1016/s0002-9378(98)70210-8. [DOI] [PubMed] [Google Scholar]
- 120.Lorentzen B, Birkeland KI, Endresen MJ, et al. Glucose intolerance in women with preeclampsia. Acta Obstet Gynecol Scand. 1998;77:22–7. [PubMed] [Google Scholar]
- 121.Kaaja R, Laivuori H, Laakso M, et al. Evidence of a state of increased insulin resistance in preeclampsia. Metabolism. 1999;48:892–6. doi: 10.1016/s0026-0495(99)90225-1. [DOI] [PubMed] [Google Scholar]
- 122.Caruso A, Ferrazzani S, Paradisi G. Insulin resistance and preeclampsia: is there a relationship? Am J Obstet Gynecol. 1999;181:768–9. doi: 10.1016/s0002-9378(99)70536-3. [DOI] [PubMed] [Google Scholar]
- 123.Nisell H, Erikssen C, Persson B, et al. Is carbohydrate metabolism altered among women who have undergone a preeclamptic pregnancy? Gynecol Obstet Invest. 1999;48:241–6. doi: 10.1159/000010191. [DOI] [PubMed] [Google Scholar]
- 124.Solomon CG, Seely EW. Brief review: hypertension in pregnancy:a manifestation of the insulin resistance syndrome? Hypertension. 2001;37:232–9. doi: 10.1161/01.hyp.37.2.232. [DOI] [PubMed] [Google Scholar]
- 125.Thadhani R, Ecker JL, Mutter WP, et al. Insulin resistance and alterations in angiogenesis: additive insults that may lead to preeclampsia. Hypertension. 2004;43:988–92. doi: 10.1161/01.HYP.0000124460.67539.1d. [DOI] [PubMed] [Google Scholar]
- 126.Emery SP, Levine RJ, Qian C, et al. Twenty-four-hour urine insulin as a measure of hyperinsulinaemia/insulin resistance before onset of pre-eclampsia and gestational hypertension. BJOG. 2005;112:1479–85. doi: 10.1111/j.1471-0528.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 127.Parretti E, Lapolla A, Dalfra M, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449–53. doi: 10.1161/01.HYP.0000205122.47333.7f. [DOI] [PubMed] [Google Scholar]
- 128.Roberts JM, Gammill H. Insulin resistance in preeclampsia. Hypertension. 2006;47:341–2. doi: 10.1161/01.HYP.0000205123.40068.84. [DOI] [PubMed] [Google Scholar]
- 129.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–9. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 130.Mazar RM, Srinivas SK, Sammel MD, et al. Metabolic score as anovel approach to assessing preeclampsia risk. Am J Obstet Gynecol. 2007;197:411.e1–5. doi: 10.1016/j.ajog.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 131.Mazaki-Tovi S, Romero R, Vaisbuch E, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat Med. 2009;37:349–63. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–71. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hauth JC, Clifton RG, Roberts JM, et al. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;204:327.e1–6. doi: 10.1016/j.ajog.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bytautiene E, Tamayo E, Kechichian T, et al. Prepregnancy obesity and sFlt1-induced preeclampsia in mice: developmental programming model of metabolic syndrome. Am J Obstet Gynecol. 2011;204:398.e1–8. doi: 10.1016/j.ajog.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 135.Lynch AM, Eckel RH, Murphy JR, et al. Prepregnancy obesity and complement system activation in early pregnancy and the subsequent development of preeclampsia. Am J Obstet Gynecol. 2012;206:428.e1–8. doi: 10.1016/j.ajog.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stekkinger E, Scholten R, van der Vlugt MJ, et al. Metabolic syndrome and the risk for recurrent pre-eclampsia: a retrospective cohort study. BJOG. 2013;120:979–86. doi: 10.1111/1471-0528.12189. [DOI] [PubMed] [Google Scholar]
- 137.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 138.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 139.Lebreton JP, Joisel F, Raoult JP, et al. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64:1118–29. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daveau M, Christian D, Julen N, et al. The synthesis of human alpha-2-HS glycoprotein is down-regulated by cytokines in hepatoma HepG2 cells. FEBS Lett. 1988;241:191–4. doi: 10.1016/0014-5793(88)81059-7. [DOI] [PubMed] [Google Scholar]
- 141.Daveau M, Davrinche C, Djelassi N, et al. Partial hepatectomy and mediators of inflammation decrease the expression of liver alpha 2-HS glycoprotein gene in rats. FEBS Lett. 1990;273:79–81. doi: 10.1016/0014-5793(90)81055-s. [DOI] [PubMed] [Google Scholar]
- 142.Wang H, Sama AE. Anti-inflammatory role of fetuin-A in injury and infection. Curr Mol Med. 2012;12:625–33. doi: 10.2174/156652412800620039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Molvarec A, Prohaszka Z, Nagy B, et al. Association of increased serum heat shock protein 70 and C-reactive protein concentrations and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol. 2007;73:172–9. doi: 10.1016/j.jri.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 144.Molvarec A, Kalabay L, Derzsy Z, et al. Preeclampsia is associated with decreased serum alpha(2)-HS glycoprotein (fetuin-A) concentration. Hypertens Res. 2009;32:665–9. doi: 10.1038/hr.2009.79. [DOI] [PubMed] [Google Scholar]
- 145.Johnstone ED, Sawicki G, Guilbert L, et al. Differential proteomic analysis of highly purified placental cytotrophoblasts in preeclampsia demonstrates a state of increased oxidative stress and reduced cytotrophoblast antioxidant defense. Proteomics. 2011;11:4077–84. doi: 10.1002/pmic.201000505. [DOI] [PubMed] [Google Scholar]
- 146.Park J, Cha DH, Lee SJ, et al. Discovery of the serum biomarker proteins in severe preeclampsia by proteomic analysis. Exp Mol Med. 2011;43:427–35. doi: 10.3858/emm.2011.43.7.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomez LM, Anton L, Srinivas SK, et al. Effects of increasedfetuin-A in human trophoblast cells and associated pregnancy outcomes. Am J Obstet Gynecol. 2012;207:e1–8. doi: 10.1016/j.ajog.2012.10.872. [DOI] [PubMed] [Google Scholar]
- 148.Gomez LM. Understanding the effects of fetuin-A in pregnancy. Med J Obstet Gynecol. 2013;1:1010. [Google Scholar]
- 149.Pedersen KO. Fetuin, a new globulin isolated from serum. Nature. 1944;154:575. [Google Scholar]
- 150.Dziegielewska KM, Brown WM, Casey SJ, et al. The complete cDNA and amino acid sequence of bovine fetuin. Its homology with alpha 2HS glycoprotein and relation to other members of the cystatin superfamily. J Biol Chem. 1990;265:4354–7. [PubMed] [Google Scholar]
- 151.Li W, Zhu S, Li J, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS One. 2011;6:e16945. doi: 10.1371/journal.pone.0016945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Oss CJ, Gillman CF, Bronson PM, et al. Opsonic properties of human serum alpha-2 hs glycoprotein. Immunol Commun. 1974;3:329–35. doi: 10.3109/08820137409061113. [DOI] [PubMed] [Google Scholar]
- 153.Lewis JG, Andre CM. Enhancement of human monocyte phagocytic function by alpha 2HS glycoprotein. Immunology. 1981;42:481–7. [PMC free article] [PubMed] [Google Scholar]
- 154.Jersmann HP, Dransfield I, Hart SP. Fetuin/alpha2-HS glycoprotein enhances phagocytosis of apoptotic cells and micropinocytosis by human macrophages. Clin Sci (Lond) 2003;105:273–8. doi: 10.1042/CS20030126. [DOI] [PubMed] [Google Scholar]
- 155.Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58:631–40. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 156.Rauth G, Poschke O, Fink E, et al. The nucleotide and partialamino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992;204:523–9. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 157.Srinivas PR, Wagner AS, Reddy LV, et al. Serum alpha 2-HSglycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993;7:1445–55. doi: 10.1210/mend.7.11.7906861. [DOI] [PubMed] [Google Scholar]
- 158.Mathews ST, Chellam N, Srinivas PR, et al. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol Cell Endocrinol. 2000;164:87–98. doi: 10.1016/s0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 159.Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–8. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 160.Stefan N, Haring HU. The role of hepatokines in metabolism. NatRev Endocrinol. 2013;9:144–52. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 161.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–85. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 162.Chaemsaithong P, Romero R, Chaiworapongsa T, et al. Fetuin-A, a negative acute phase protein reactant, is decreased in preeclampsia. Am J Obstet Gynecol. 2014 (Submitted) [Google Scholar]
- 163.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 164.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 165.Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 166.Gonzalez RP, Gomez RM, Castro RS, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–65. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 167.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 168.Douglas B, Maechler M, Bolker B. lme4: Linear mixed effects models using S4 classes. R package version 0.999999-0. 2012 Available from: http://CRAN.R-project.org/package1/4lme4.
- 169.Vince GS, Starkey PM, Austgulen R, et al. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 170.Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38:89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 171.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 172.Teran E, Escudero C, Moya W, et al. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with preeclampsia. Int J Gynaecol Obstet. 2001;75:243–9. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 173.Serin IS, Ozcelik B, Basbug M, et al. Predictive value of tumornecrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2002;100:143–5. doi: 10.1016/s0301-2115(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 174.Velzing-Aarts FV, Muskiet FA, van der Dijs FP, et al. High serum interleukin-8 levels in afro-caribbean women with pre-eclampsia. Relations with tumor necrosis factor-alpha, duffy negative phenotype and von Willebrand factor. Am J Reprod Immunol. 2002;48:319–22. doi: 10.1034/j.1600-0897.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 175.Kocyigit Y, Atamer Y, Atamer A, et al. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 2004;19:267–73. doi: 10.1080/09513590400018108. [DOI] [PubMed] [Google Scholar]
- 176.Afshari JT, Ghomian N, Shameli A, et al. Determination of interleukin-6 and tumor necrosis factor-alpha concentrations in Iranian-Khorasanian patients with preeclampsia. BMC Pregn Childbirth. 2005;5:14. doi: 10.1186/1471-2393-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang X, Huang H, Dong M, et al. Serum and placental interleukin-18 are elevated in preeclampsia. J Reprod Immunol. 2005;65:77–87. doi: 10.1016/j.jri.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 178.Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 179.Szarka A, Rigo J, Jr, Lazar L, et al. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xie C, Yao MZ, Liu JB, et al. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011;56:550–9. doi: 10.1016/j.cyto.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 181.Ozler A, Turgut A, Sak ME, et al. Serum levels of neopterin, tumor necrosis factor-alpha and Interleukin-6 in preeclampsia: relationship with disease severity. Eur Rev Med Pharmacol Sci. 2012;16:1707–12. [PubMed] [Google Scholar]
- 182.Soto E, Romero R, Richani K, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med. 2010;23:646–57. doi: 10.3109/14767050903301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saito S, Umekage H, Sakamoto Y, et al. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 184.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, et al. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;86:165–70. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 185.Darmochwal-Kolarz D, Rolinski J, Leszczynska-Goarzelak B, et al. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol. 2002;48:381–6. doi: 10.1034/j.1600-0897.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 186.Rein DT, Schondorf T, Gohring UJ, et al. Cytokine expression in peripheral blood lymphocytes indicates a switch to T(HELPER) cells in patients with preeclampsia. J Reprod Immunol. 2002;54:133–42. doi: 10.1016/s0165-0378(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 187.Luppi P, Deloia JA. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin Immunol. 2006;118:268–75. doi: 10.1016/j.clim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 188.Naccasha N, Gervasi MT, Chaiworapongsa T, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–23. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 189.Boij R, Svensson J, Nilsson-Ekdahl K, et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68:258–70. doi: 10.1111/j.1600-0897.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 190.Peracoli JC, Bannwart-Castro CF, Romao M, et al. High levels ofheat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol. 2013;100:129–34. doi: 10.1016/j.jri.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 191.Jahnen-Dechent W, Schinke T, Trindl A, et al. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- 192.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–66. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moe SM, Reslerova M, Ketteler M, et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD) Kidney Int. 2005;67:2295–304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 194.Roos M, Richart T, Kouznetsova T, et al. Fetuin-A and arterial stiffness in patients with normal kidney function. Regul Pept. 2009;154:39–43. doi: 10.1016/j.regpep.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 195.Ford ML, Tomlinson LA, Smith ER, et al. Fetuin-A is an independent determinant of change of aortic stiffness over 1 year in non-diabetic patients with CKD stages 3 and 4. Nephrol Dial Transplant. 2010;25:1853–8. doi: 10.1093/ndt/gfp723. [DOI] [PubMed] [Google Scholar]
- 196.Jung JY, Hwang YH, Lee SW, et al. Factors associated with aortic stiffness and its change over time in peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25:4041–8. doi: 10.1093/ndt/gfq293. [DOI] [PubMed] [Google Scholar]
- 197.Caglar K, Yilmaz MI, Saglam M, et al. Endothelial dysfunction and fetuin A levels before and after kidney transplantation. Transplantation. 2007;83:392–7. doi: 10.1097/01.tp.0000251647.72673.c7. [DOI] [PubMed] [Google Scholar]
- 198.Caglar K, Yilmaz MI, Saglam M, et al. Short-term treatment with sevelamer increases serum fetuin-a concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin J Am Soc Nephrol. 2008;3:61–8. doi: 10.2215/CJN.02810707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tihtonen KM, Koobi T, Uotila JT. Arterial stiffness in preeclamptic and chronic hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 2006;128:180–6. doi: 10.1016/j.ejogrb.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 200.Kaihura C, Savvidou MD, Anderson JM, et al. Maternal arterial stiffness in pregnancies affected by preeclampsia. Am J Physiol Heart Circ Physiol. 2009;297:H759–64. doi: 10.1152/ajpheart.01106.2008. [DOI] [PubMed] [Google Scholar]
- 201.Hausvater A, Giannone T, Sandoval YH, et al. The association between preeclampsia and arterial stiffness. J Hypertens. 2012;30:17–33. doi: 10.1097/HJH.0b013e32834e4b0f. [DOI] [PubMed] [Google Scholar]


