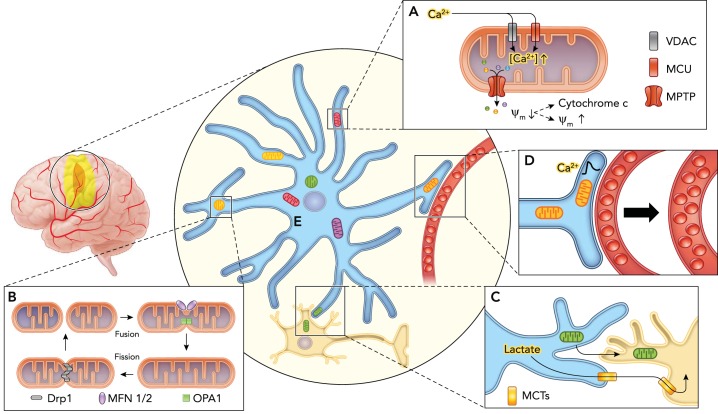
FIGURE 1.
Schematic illustrating the response of astrocytic mitochondria to ischemia
A: loss of oxygen and glucose leads to increased cytosolic Ca2+ concentrations that drives excessive accumulation of Ca2+ into the mitochondria via voltage-dependent anion channels (VDACs) and mitochondrial calcium uniporters (MCUs), triggering opening of the large mitochondrial permeability transition pore (MPTP). This allows the indiscriminate passage of small solutes out of the mitochondria causing dissipation of the mitochondrial membrane potential (ψm), which can culminate in release of cytochrome c and cellular apoptosis or membrane potential recovery with cell survival. B: mitochondria undergo morphological and network architectural changes in response to ischemia. They can adopt rounder discrete shape via fission mediated by dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1), or form elongated, tubular interconnected network via fusion mediated by the outer membrane GTPases mitofusin-1 and -2 (MFN1/MFN2) and the inner membrane protein optic atrophy 1 (OPA1). C: astrocytes can promote neuronal survival by providing lactate as an energy substrate via monocarboxylate transporters (MCT) to neurons in the setting of impaired oxidative phosphorylation, in addition to directly donating functional mitochondria via a CD38-dependent mechanism. D: astrocyte mitochondria are enriched within vascular endfeet and may play a central role in neurovascular coupling by regulating Ca2+ signals. E: mitochondria are heterogeneous in structure and function, which may contribute to astroglial diversity. A subpopulation of astrocytic mitochondria may also be selectively resilient to ischemia.