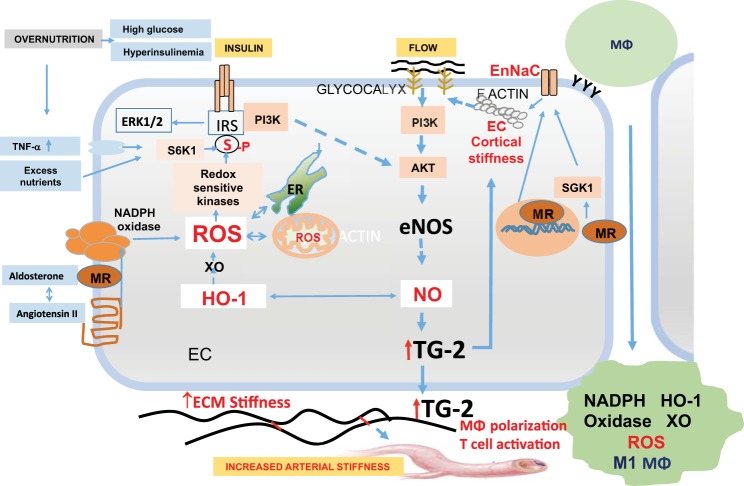
Fig. 2.
Endothelial cell (EC)-macrophage-vascular smooth muscle cell interactions in obesity-associated arterial stiffness. Impaired insulin metabolic signaling in ECs leads to decreased activation of endothelial nitric oxide (NO) synthase (eNOS) and NO bioavailability. Increase in endothelial Na+ channel (EnNaC) activity occurs due to mineralocorticoid receptor (MR)-induced activation of serum/glucocorticoid regulated kinase 1 (SGK1) and increased EnNaC expression, which result in increased influx of Na+ and polymerization of F actin, leading to endothelial cortical stiffness. This leads to impaired flow-mediated release of NO. Increased oxidative stress due to RAAS-induced activation of NADPH oxidase, xanthine oxidase, and mitochondrial oxidative stress results in increased destruction of NO. eNOS uncoupling leads to further decreases in bioavailable NO. Decreased bioavailable NO causes increased expression of adhesion molecules on ECs, favoring monocyte recruitment. Decreased bioavailable NO also results in macrophage (MΦ) activation, M1 macrophage polarization, and oxidative stress. This leads to further destruction of NO. Decreased bioavailable NO results in transglutaminase 2 (TG2) activation, which promotes collagen cross-linking and causes cortical stiffness, which impairs flow-mediated NO production. Decreased heme oxygense 1 (HO-1) activity further contributes to the decrease in bioavailable NO, thereby promoting arterial stiffness. ECM, extracellular matrix; ERK1/2, extracellular-regulated kinase 1/2; IRS-1, insulin receptor substrate-1; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen species; S6K1, S6 kinase 1.