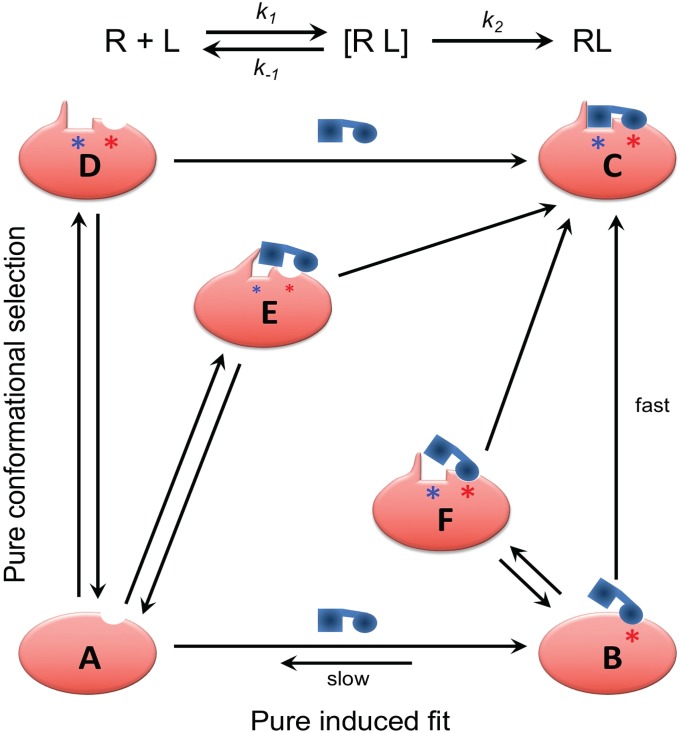
Fig. 4.
Continuum of mechanisms for binding to cryptic sites. In an induced fit binding mechanism, the initial encounter of the ligand with the unbound protein, A, is “sticky”: that is, dissociation of the initial encounter complex B, with rate k-1, is slow compared with conversion to the final, stable complex C, with rate constant k2, allowing time for the ligand-induced conformational change in the protein to take place within the lifetime of the encounter complex. For the alternative, conformational selection mechanism, most encounters between the ligand and protein are nonproductive, with the ligand rapidly dissociating. Only when the initial encounter occurs with one of the small fraction of protein molecules in which the cryptic site (shown by a blue asterisk) is fully formed (D) will reaction proceed to give a stable complex. Intermediate situations can be conceived where dissociation of the initial encounter complex occurs at a rate comparable with that for partitioning forward to form a stable complex. One hybrid mechanism involves an initial encounter with a form of the protein, E, in which the cryptic site is only partially formed through spontaneous conformational fluctuations, requiring the presence of the ligand to promote conversion to the final complex. Another is when ligand binds to a noncompetent conformation of the protein in a relatively sticky manner but must then wait for one or more adjacent subpockets to open through stochastic conformational fluctuations (F) before forming the final complex. We propose that mechanisms toward the induced fit end of this mechanistic spectrum (Bottom Right) are more likely when there is a strong hot spot (shown by a red asterisk) adjacent to the cryptic site even in protein conformers in which the cryptic site itself is occluded in all known unbound structures, a situation that describes over 50% of the examples present in the CryptoSite protein set.