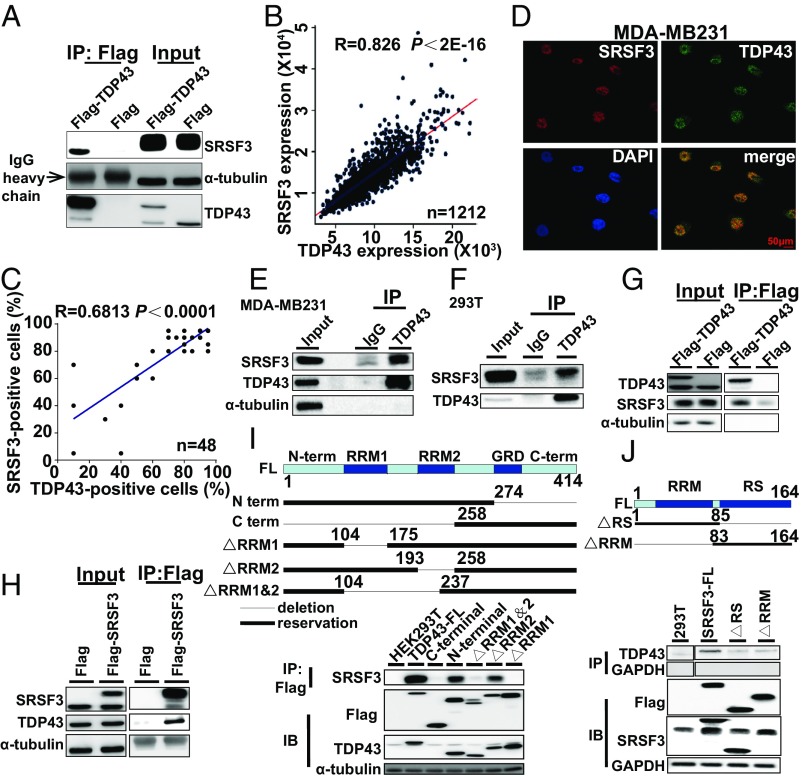
Fig. 5.
TDP43 interacts with SRSF3 in breast cancer cells. (A) Cellular extracts from MDA-MB231 cells stably expressing Flag (control) or Flag-TDP43 were immunopurified with anti-Flag affinity beads. Immunocomplexes were then immunoblotted using antibodies against the indicated proteins. (B) Analysis of public datasets for expression correlation between TDP43 and SRSF3. (C) Analysis of expression correlation between TDP43 and SRSF3 by immunohistochemical staining of tissue chip data. (D) Subcellular localization of TDP43 (green) and SRSF3 (red) in MDA-MB231 cells determined by immunofluorescence by confocal fluorescence microscopy. (E and F) Endogenous immunopurification with TDP43 antibody in MDA-MB231 (E) and 293T (F) cells. (G and H) Flag-TDP43 pulled down with SRSF3 (G) or Flag-SRSF3 pulled down with TDP43 in HEK293T cells (H). (I and J, Upper) Structures of TDP43 (I) and SRFS3 (J) and their mutants used in immunoprecipitation pull-down assays. TDP43 protein (I) exhibits an N-terminal domain (N-term), two RRMs (RRM1 and RRM2), a glycine-rich domain (GRD), and a C-terminal domain (C-term); whereas SRSF3 full-length protein (J) contains an RRM and an SR-rich domain (RS). (Lower) Flag-mutants of TDP43 pulled down with SRSF3 (I) or Flag-mutants of SRSF3 pulled down with TDP43 (J) from HEK293T cells.