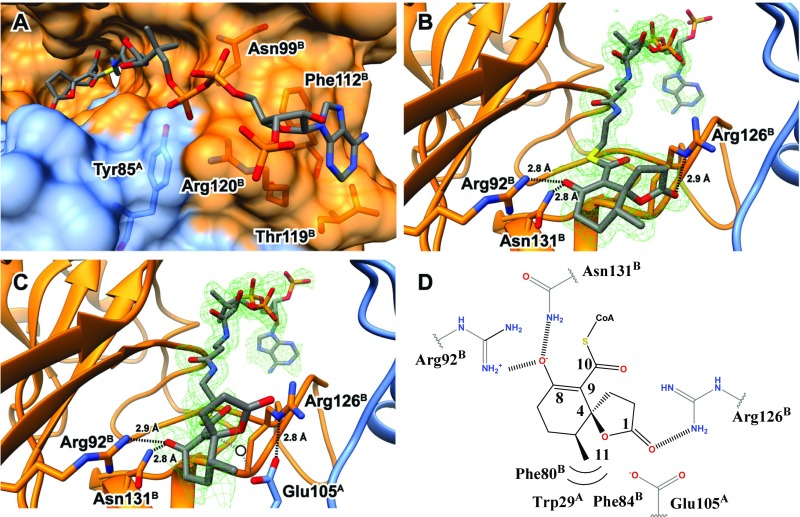
Fig. 5.
Structure of IpdAB⋅COCHEA-CoA. (A) Lactonized COCHEA-CoA binds along a long cleft between IpdA (blue) and IpdB (orange). Residues proposed to be involved in binding of the CoA moiety are shown in ball and stick. (B) Active site of IpdABRHA1 E105AA⋅COCHEA-CoA complex displaying putative catalytic residues in ball and stick. The mFo-DFc substrate omit map contoured at 3.0 σ is shown in green mesh. (C) Active site of the WT IpdABRHA1⋅COCHEA-CoA complex displaying putative catalytic residues in ball and stick. The mFo-DFc substrate omit map contoured at 3.0 σ is shown in green mesh. Potential position of catalytic water we suggest would be bound in the unlactonized form of substrate is represented by a white circle. (D) Diagram illustrating residues predicted to make contacts with the lactonized COCHEA-CoA in the IpdABRHA1 active site.