Fig. 1.
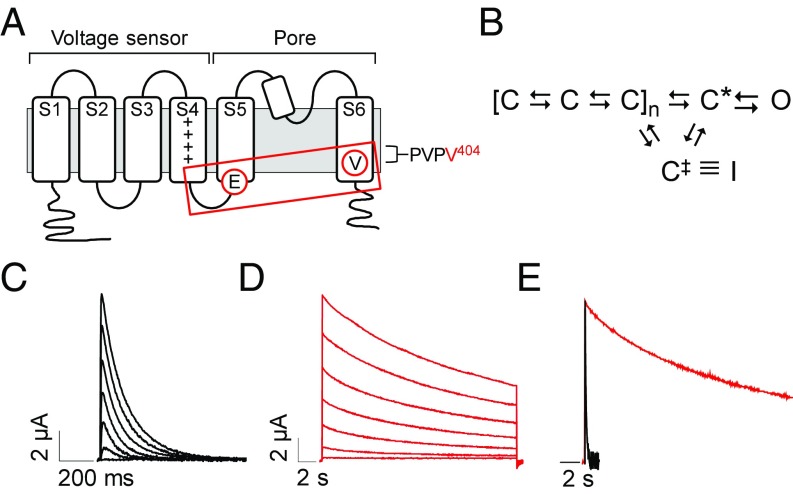
V404M significantly slows macroscopic current decay. (A) Membrane topology of Kv4.2 subunit is shown. The voltage sensor (transmembrane segments S1–S4) and pore (S5, S6, and reentrant loop) domains are bracketed. Control of channel activity by voltage is primarily due to positively charged residues (+) in the S4 segment (19, 20). Circled residues indicate the approximate locations of V404 in S6 immediately after the PVP motif and E323 in the S4–S5 linker. Voltage sensor is coupled to the pore gate by interactions between S4–S5 linker and S6 gate residues, including V404 (boxed). (B) Highly simplified gating scheme shows essential features of CSI based on available evidence (10, 21–25). In response to depolarization, the voltage sensor domain undergoes transitions between closed states to reach an activated conformation, C*, that is permissive for pore opening. The channel inactivates preferentially from partially activated closed conformations or C*. For CSI, the voltage sensor transitions to an alternative conformation, C‡, that corresponds to the inactivated state (I) if the pore gate is closed. Reflecting evidence that open channels must close to inactivate, there is no direct pathway between the open and inactivated states (25, 26). (C and D) Representative current traces from (C) wild-type Kv4.2 (black traces) or (D) V404M (red traces). In this figure and subsequent figures, wild-type and mutant Kv4.2 constructs were coexpressed with KChIP3a. Note that V404M currents are shown on a compressed time base. Currents were evoked by pulsing from −100 mV to voltages from −60 to +60 mV. Traces shown were obtained at 20-mV increments. (E) Representative wild-type (black) and V404M (red) currents recorded at +20 mV were scaled to the same amplitude and overlaid. Traces are shown on the same time base.