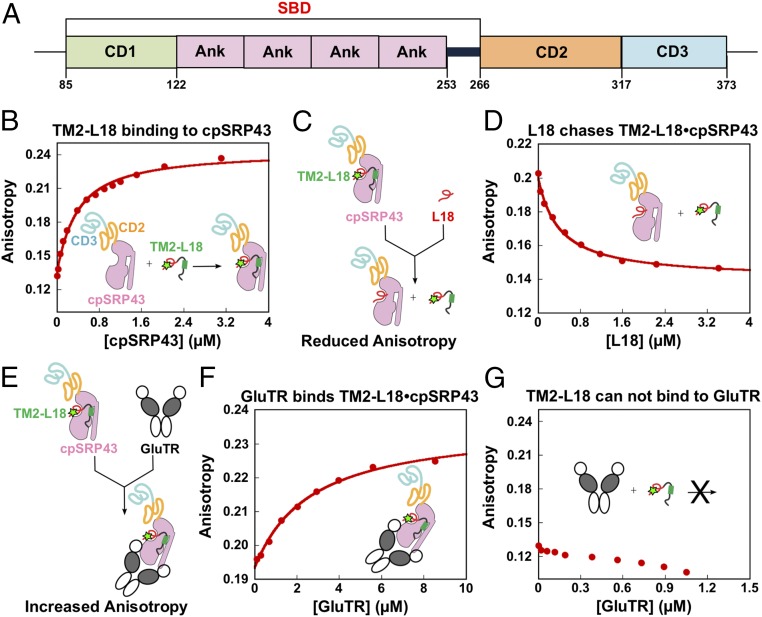
Fig. 4.
GluTR and LHCb5 bind cpSRP43. (A) Schematic overview of cpSRP43 domains. (B) Binding of the loop1-TM2-L18 fragment of LHCb5 to cpSRP43. Binding was measured by monitoring changes in the fluorescence anisotropy of LHCb5 (G162C) labeled with fluorescein at position 162. The data were fit to SI Appendix, Eq. 1 and gave a Kd value of 338 nM. (C and E) Scheme illustrating the effects of a competitor (L18 in D) or cobinder (GluTR in F) on the fluorescence anisotropy of the LHCb5 fragment prebound to cpSRP43-SBD. The observed effect of the L18 peptide (D) or GluTR (F) on the fluorescence anisotropy of the LHCb5 fragment prebound to cpSRP43 is shown. The data in D were fit to the competition model described by SI Appendix, Eq. 2 and gave a value of 1 μM. The data in F were fit to SI Appendix, Eq. 1 and gave a Kd value of 2.6 μM. The SDs of the Kd and values were estimated to be ±10% based on at least two measurements (technical replicates). In G, GluTR does not bind to the fluorescein-labeled LHCb5 fragment.