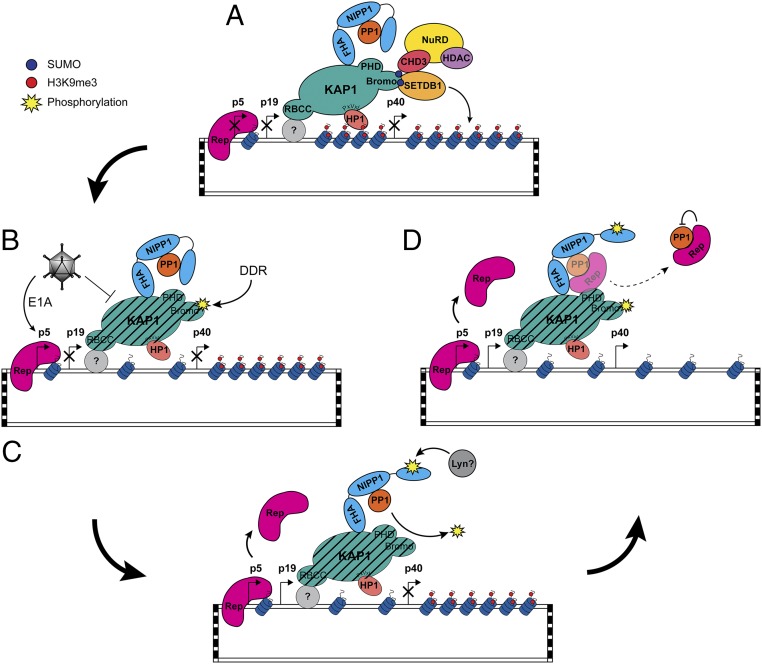
Fig. 5.
Model for the release of AAV2 from KAP1-mediated latency. (A) Incoming AAV2 genomes undergo second-strand synthesis, concatamerization, and chromatinization upon nuclear entry. KAP1 is recruited to the rep ORF via an unknown binding partner where it forms a scaffold for the recruitment of SETDB1 and CHD3, leading to the methylation of AAV2-associated histones. NIPP1 is recruited to KAP1 via the FHA domain, where it serves to tether inactive PP1 to KAP1. (B and C) Upon coinfection, KAP1 repression is partially lifted through several potential mechanisms: helper-mediated degradation of KAP1 as we have observed, interference with KAP1 SUMOylation as observed by others (51), and/or phosphorylation of KAP1-S824 triggered by initial helper- or AAV-mediated stress response (B), allowing up-regulation of rep by Ad5 E1A (C). Unknown cellular factors/RNA binding inactivate NIPP1, allowing PP1 to restore baseline levels of p-KAP1-S824. (D) Sequestration of PP1 by Rep sustains enhanced levels of phosphorylated KAP1-S824 to support lytic replication.