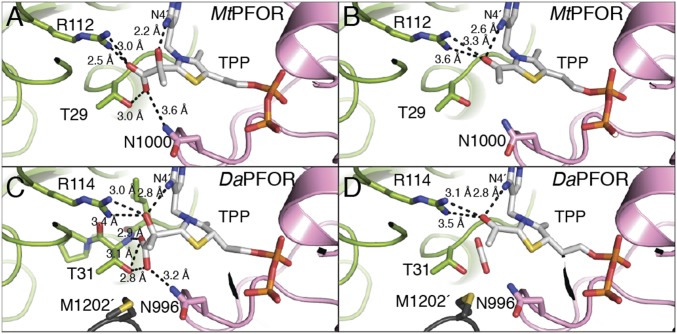
Fig. 3.
Active-site comparisons for intermediates in MtPFOR and DaPFOR. (A) Lactyl-TPP intermediate in MtPFOR. The thiazolium ring is planar, and the carbon–carbon bond between C2 of TPP and the lactyl group is 1.6 Å as expected for a carbon–carbon bond. (B) Acetyl-TPP adduct in MtPFOR. The thiazolium ring is planar, and the carbon–carbon bond between C2 of TPP and the acetyl group is 1.5 Å as expected for a carbon–carbon bond. (C) Lactyl-TPP intermediate captured in the structure of DaPFOR [PDB ID code 2C3P (17)] has a nonplanar aromatic thiazolium ring. The carbon–carbon bond between C2 of TPP and the lactyl group is 1.9 Å. (D) Putative HE-TPP intermediate in the structure of DaPFOR [PDB ID code 2C3Y (17)] has a nonplanar aromatic thiazolium ring. The carbon–carbon bond between C2 of TPP and the acetyl group is long for a carbon–carbon bond at 1.8 Å.