Fig. 4.
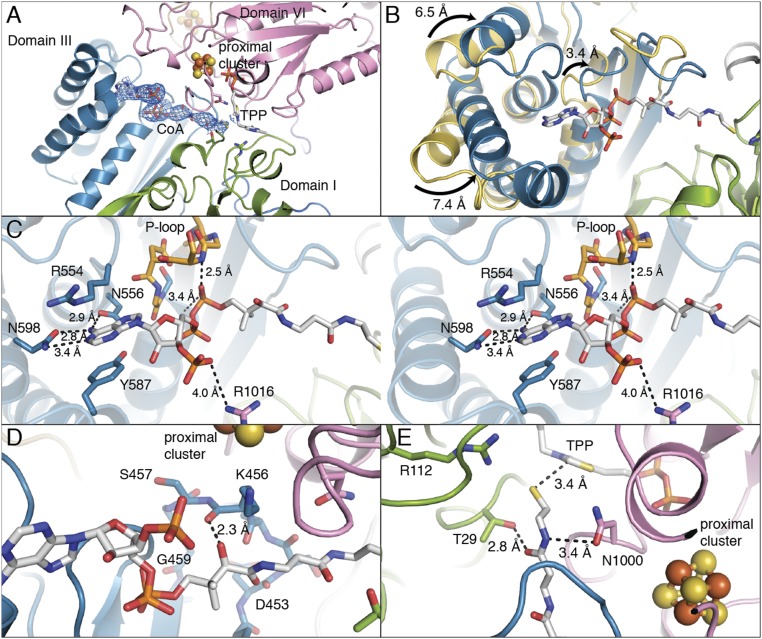
Domain III of MtPFOR is a CoA-binding domain. (A) The pyrophosphate and adenine end of CoA (in sticks) binds to domain III (teal), and the CoA molecule extends into the active site, which is composed of domains I (green) and VI (pink). The 2Fo − Fc composite-omit electron density for CoA is shown in blue mesh that is contoured to 1.0 σ. (B) Structural superposition of domain III from MtPFOR with (teal) and without (yellow) CoA bound. The overall Cα rmsd of domain III (residues 415–630) is 4.9 Å with parts of the domain moving up to 7.4 Å. (C) Stereo views of domain III of MtPFOR with CoA bound illustrate residues anchoring adenosine moiety and pyrophosphate of CoA. Domain III of MtPFOR with CoA bound is colored teal; CoA and CoA binding residues of MtPFOR are shown as sticks. Residues 421–426 form P-loop (orange), which binds the pyrophosphate moiety of CoA by the backbone of P-loop and the dipole of the subsequent alpha helix. Asn556 and Asn598 form hydrogen bonds with adenine of CoA. Arg554 and Tyr587 bind the adenine by cation–pi interaction and pi–pi stacking interactions. Arg1016 forms a charge–charge interaction with 3′-phospho group of CoA. (D) The carboxyl group on the pantothenate moiety of CoA forms a hydrogen bond with the carbonyl backbone of Lys456. (E) Thr29 and Asn1000, which form hydrogen bonds with lactyl-TPP intermediates (Fig. 3A), also form hydrogen bonds with the cysteamine moiety of CoA.