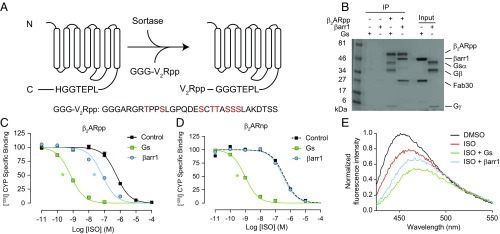
Fig. 3.
Sortase ligation of a phosphopeptide onto the β2AR restores its allosteric interaction with β-arrestin1. (A) Cartoon schematic of sortase ligation method. A synthetic phosphopeptide (pp) derived from the vasopressin-2-receptor (V2R) with three N-terminal glycine residues (GGG-V2Rpp) is ligated onto receptors containing a C-terminal LPETGGH recognition motif. In the sequence of GGG-V2Rpp below the schematic, phosphorylated residues are highlighted in red. (B) Coomassie-stained gel showing the coimmunoprecipitation of heterotrimeric Gs and β-arrestin1 (βarr1) with isoproterenol (ISO)-bound, phosphopeptide-ligated FLAG-β2AR (β2ARpp). Fab30 binds specifically to V2Rpp-bound βarr1 (10). Loading controls represent 10% of input. (C and D) Competition binding experiments using radiolabeled [125I]-cyanopindolol (CYP) with HDLs containing (C) β2ARpp or (D) β2AR ligated to a nonphosphorylated version of the V2R peptide (β2ARnp). Gs increases the affinity of ISO for both β2ARpp and β2ARnp HDLs (log IC50: −9.15 ± 0.03, −9.02 ± 0.04, respectively) compared with no transducer (log IC50: −6.24 ± 0.04, −6.42 ± 0.09, respectively), but βarr1 only increases ISO affinity for β2ARpp HDLs (log IC50: −7.14 ± 0.07) and not β2ARnp HDLs (log IC50: −6.49 ± 0.04). Data shown in C and D are the mean of at least three independent experiments, with error bars representing SE, and asterisks (*) indicate a log IC50 value significantly different from the control curve (P < 0.05, one-way ANOVA). (E) The effects of ISO on the HDL-β2ARpp-bimane fluorescence emission spectrum are enhanced by Gs and βarr1. Data shown are representative of three independent experiments.