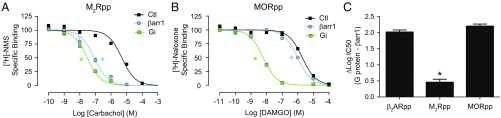
Fig. 5.
The allosteric enhancement of agonist binding induced by β-arrestin1 varies among different receptors. (A) Competition binding experiments with sortase-ligated M2Rpp HDLs, using [3H]-N-methyl-scopolamine (NMS) as the tracer. Heterotrimeric Gi (100 nM, log IC50: −7.51 ± 0.06) and β-arrestin1 (βarr1) (1 μM, log IC50: −7.06 ± 0.08) increase the affinity of the agonist carbachol to a similar extent (no transducer, log IC50: −5.31 ± 0.09). (B) Competition binding experiments with sortase-ligated MORpp HDLs, using [3H]-naloxone as the tracer. Gi (1 μM, log IC50: −8.22 ± 0.05) increases the affinity of the agonist DAMGO to a far greater extent than βarr1 (1 μM, log IC50: −6.02 ± 0.05) (no transducer, log IC50: −5.71 ± 0.06). Data in A and B are the mean of three independent experiments, with error bars representing SE, and asterisks (*) indicate a log IC50 value significantly different from the control curve (P < 0.05, one-way ANOVA). (C) Comparison of the difference in agonists’ log IC50 values in the presence of their cognate G proteins versus βarr1 for sortase-ligated β2ARpp (Fig. 3C), M2Rpp (A), and MORpp (B) HDLs.