Abstract
The brain is the most common site of first metastasis for patients with HER2-positive breast cancer treated with HER2-targeting drugs. However, the development of effective therapies for breast cancer brain metastases (BCBMs) is limited by an incomplete understanding of the mechanisms governing drug sensitivity in the central nervous system. Pharmacodynamic data from patients and in vivo models suggest that inadequate drug penetration across the ‘blood-tumor’ barrier is not the whole story. Using HER2-positive breast cancer brain metastases as a case study, we highlight recent data from orthotopic brain metastasis models that implicates brain-specific drug resistance mechanisms in BCBMs and suggests a translational research paradigm to guide drug development for treatment of BCBMs.
Introduction
Brain metastases represent a significant clinical challenge for the treatment of patients with human epidermal growth factor receptor 2 (HER2) positive (amplified) breast cancer(1). Current standard of care for breast cancer brain metastases (BCBMs) involves surgical resection of solitary lesions and radiotherapy for multiple lesions leading to median survival for good performance patients of up to 23.5 months(2). However, there is no current consensus on subsequent therapy for those with intracranial progression and effective, FDA-approved, drugs for this indication remain an area of unmet need(3).
While the incidence of BCBMs at diagnosis of advanced disease for patients with HER2-positive breast cancer is approximately 11%, brain metastases eventually affect up to 50% of patients with metastatic disease(4–8). Among patients who received adjuvant trastuzumab in the HERA study, the brain made up a larger proportion of the sites of initial relapse in the trastuzumab arm compared to control(8). These data suggest that in the era of adjuvant HER2-directed systemic therapy, patients experience extracranial disease control while the brain represents a ‘sanctuary site’ where systemic HER2-directed therapies are less effective (9).
The discordant efficacy of a drug at different metastatic sites within the same patient can be ascribed, in principle, to three causes: 1) wrong drug, i.e. the molecule is unable to act on its molecular target; 2) wrong place, i.e. the molecule is not delivered to its molecular target; 3) wrong target, i.e. target inhibition is insufficient to trigger cancer cell death. Therefore, in the case of HER2-targeted drugs that inhibit tumor growth at extracranial sites, failure to be effective against BCBMs in patients can either occur from 2) or 3) i.e. either the drug does not reach the target or target inhibition is insufficient to cause cell killing.
Failure of cytotoxic drugs to kill brain metastases has been widely attributed to inadequate drug penetration into the brain parenchyma through the blood-brain barrier (BBB)(10,11). However, detailed studies of drug delivery / drug efficacy in patients with brain metastases or patient-derived xenograft mouse models instead suggest that BCBM survival occurs despite adequate delivery and activity of cytotoxic agents(12–14).
In this Perspective, we propose that the dominant concept used to explain drug resistance in HER2-positive BCBMs: inadequate drug delivery to the tumor, is not borne out by evidence from animal and human studies of HER2-positive BCBMs. We go on to examine compelling pre-clinical evidence that suggests drug resistance in HER2-positive BCBMs is, in part, cued by specific signals from the brain microenvironment (15,16). These data suggest new approaches to more faithfully model drug resistance in the laboratory and highlight the need to design clinical trials that aim to exploit the unique vulnerabilities of brain metastases.
Discordant drug sensitivity
A discordant intracranial versus extracranial tumor response to anti-HER2 therapy is well recognized in clinical practice and clinical trials(17). A meta-analysis of HER2-positive breast cancer patients (n=4921) found that patients treated with adjuvant trastuzumab were more likely than untreated patients to develop a first metastatic relapse in the central nervous system (2.56% vs. 1.94%)(9). In addition, CNS metastases made up a greater proportion of first metastatic sites (16.94% vs. 8.33%) in trastuzumab treated patients. This phenomenon of extracranial disease control and intracranial disease progression was also seen in two trials of patients with metastatic HER2-positive breast cancer treated with afatinib, an EGFR/HER2 multi-kinase inhibitor(18)
In a phase 2 study of neratinib (a dual EGFR/HER2 kinase inhibitor) monotherapy, patients with prior trastuzumab treatment had lower objective response rates (ORR) (24% vs 56%) and worse median progression-free survival (PFS) (22.3 vs. 39.6 weeks)(19). While neratinib was recently added to the adjuvant armamentarium for patients with HER2-positive breast cancer, a phase 2 study of neratinib in 40 previously treated HER2-positive BCBM patients had an ORR of 8% with a median PFS of 1.9 months(20,21). Thus, response rates and median PFS of single-agent neratinib in patients with CNS metastases are more akin to patients with previous trastuzumab exposure, suggesting possible intrinsic tumor resistance. While it has been assumed that small molecules (e.g. neratinib, molar mass: 557.04 g/mol) would be more likely to cross the BBB than monoclonal antibodies (e.g. trastuzumab, molar mass: 145531.5 g/mol) a discordant intracranial and extracranial drug response associated with both drug classes suggests that additional drug resistance mechanisms may be at play(22,23). Intriguingly, the combination of neratinib + capecitabine was found to have a CNS ORR of 49%, suggesting that BCBM-specific resistance to HER2 inhibition may be overcome by combination therapy(24). A summary of intracranial response rates to selected targeted therapies in patients with BCBMs is shown in Table 1 and recently reviewed by Lin and colleagues (1).
Table 1.
Clinical efficacy of targeted therapies in breast cancer brain metastases
| Regimen | Target | Breast cancer subtypes |
CNS response rate | Reference |
|---|---|---|---|---|
| Afatinib | HER2/EGFR | HER2+ | 0% | Cortes et al, Lancet Oncol. 2015; 16:1700–1710 |
| Neratinib | HER2/EGFR | HER2+ | 8% | Freedman et al, J Clin Oncol. 2016; 34:945–952 |
| Neratinib + capecitabine | HER2/EGFR, antimetabolite | HER2+ | 49% | Freedman et al, J Clin Oncol. 35, no. 15_suppl (May 2017) 1005–1005 |
| Tucatinib | HER2 | HER2+ | 7% | Metzger et al, J Clin Oncol. 2016; 32:5s (suppl; abstr TPS660) |
| Tucatinib +/− capecitabine or trastuzumab | HER2, antimetabolite | HER2+ | 42% | Hamilton et al, Poster P5-20-1, SABCS 2017 |
| Lapatinib | HER2 | HER2+ | 6% | Lin et al, Clin Cancer Res. 2009; 15:1452–1459. |
| Lapatinib + capecitabine | HER2, antimetabolite | HER2+ | 18–38% (pre-treated); 66% (untreated) | Lin et al, J Neurooncol. 2011; 105:613–620. Bachelot et al, Lancet Oncol. 2013; 14:64–71. |
| Buparlisib + trastuzumab | PI3K + HER2 | HER2+ | 11% | Pistilli et al., Breast Cancer Res Treat (2017). |
| Everolimus + trastuzumab + vinorelbine | mTOR + HER2 + anti-mitotic | HER2+ | 4% | Anders et al, J Clin Oncol 35, 2017 (suppl; abstr 1011) |
A blood-tumor ‘barrier’ to HER2-targeted drugs?
The normal BBB is a neurovascular unit composed of highly specialized mural and supportive cells (astrocytes, microglia, neurons, pericytes, and endothelial cells), with high electrical resistance, low permeability (due to tight junctions) and armed with efflux pumps (p-glycoprotein, breast cancer resistant protein and others) that dynamically regulate the transport of macromolecules and cells into and out of the brain parenchyma(25–27). The movement of gases, water, electrolytes, macromolecules and xenobiotics is also regulated by the conditional expression of carrier-mediated transport proteins at the blood-brain interface(27).
Failure of cytotoxic drugs to kill brain metastases has been widely attributed to inadequate drug penetration into the brain parenchyma through the BBB(10). This conclusion is based on data from in vitro drug diffusion properties, normal rodent brain, or compared serum and cerebrospinal fluid (CSF) drug concentrations with the assumption that these accurately model conditions in patients with brain metastases(10,28). However, evidence suggests that the BBB, and its permeability, is significantly altered in the presence of metastatic tumor (called the blood-tumor-barrier, BTB). For example, in the presence of BCBMs the BTB is characterized by the increased presence of desmin-positive pericytes leading to local variations in drug permeability (left panel, Figure 1)(29,30). Similarly, the increased chemosensitivity of the WNT subtype of medulloblastoma (compared to the SHH subtype) has been attributed to increased vascular fenestrations in the tumor-associated vasculature(25). A recent study using a brain-tropic breast cancer cell line, MDA-MB-231, (with corroborating data from patients with leptomeningeal metastases), found that cancer-cell expression of complement component 3a disrupts the blood-CSF barrier, facilitating the survival of breast cancer cells as leptomeningeal metastases(31). Thus, the factors determining drug concentrations reaching a BCBM are unlikely to be a simple function of a drug’s molecular weight and more likely determined by the functional properties of the blood-brain barrier as altered by growing tumor.
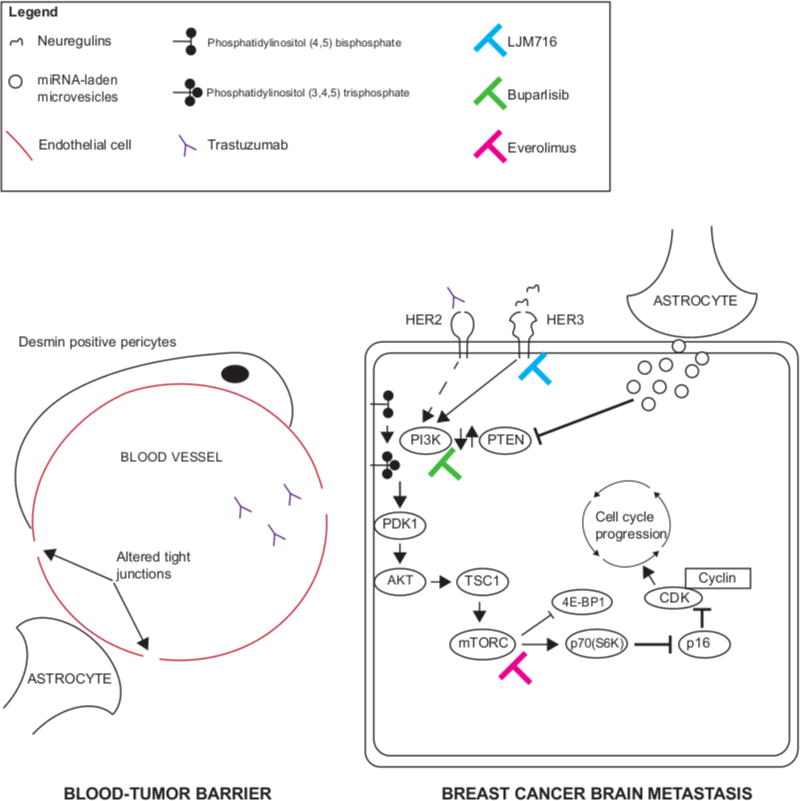
Figure 1. Brain-specific drug resistance mechanisms offer novel therapeutic targets for HER2-positive breast cancer brain metastases.
(Left panel) The blood-tumor-barrier associated with BCBMs has increased permeability mediated by desmin-positive pericytes and altered tight junctions. Although this allows trastuzumab to penetrate the brain parenchyma, inhibition of HER2 alone (Right panel, dashed arrow) is counteracted by brain-specific resistance mechanisms (see text for details). (Right panel) Brain-specific drug resistance mechanisms in BCBMs include loss of PTEN expression and activation of the PI3K-AKT-mTOR pathway as well as activation of parallel signaling pathways via neuregulin-HER3 axis. Drug inhibition of these targets (colored bar-headed line) shows promising efficacy in pre-clinical models.
CSF/serum drug concentration ratio has been used to predict the likelihood of drug penetration into the metastatic tumor bed, and from there, extrapolated to predict likelihood of efficacy (10,32). However, reported CSF/serum drug concentration ratios vary from 0–1 depending on the drug, route of administration (intravenous vs. intra-arterial), model system used, and whether human subjects had intracranial disease or not(10). In addition, while CSF drug concentrations may be dramatically influenced by changes to the BTB by either tumor-intrinsic mechanisms or by treatment e.g. radiation, it is not clear whether this increases drug delivery in the tumor bed or, more importantly, whether this translates to more effective tumor killing(27,33).
Careful studies with radiolabeled trastuzumab in both patients and mouse models have provided an alternate approach to measuring drug delivery to the tumor vasculature and across the BTB. In a mouse model of BCBMs using MMTV-human HER2 transgenic lines Fo2-1282 and Fo5, uptake of 89Zr-labeled trastuzumab was equivalent in tumors implanted in the mouse brain compared to the mammary fat pad (when compared to 89Zr-anti-STEAP1 control)(34). The authors specifically tested whether 89Zr-labeled trastuzumab penetration into the tumor graft site was merely a function of local trauma from surgery by performing sham surgery on the contralateral hemisphere and found that trastuzumab antibody concentrations in the brain tumor tissue (by ELISA) were 1000-fold less at the sham surgery site. In a study of radio-labelled trastuzumab in 6 patients with HER2-positive breast cancer, 2 patients with CNS metastases had evidence of 64Cu-DOTA-trastuzumab uptake by PET in radiologically (MRI) defined lesions (35).
Studies using trastuzumab conjugated to emtansine, a cytotoxic anti-microtubule agent, [Ado-trastuzumab Emtansine (TDM1)], provide additional evidence that BCBM drug resistance is not simply a function of poor drug penetrance across the BTB. HER2-positive cell line xenograft BCBM mouse models (established by intra-carotid injection) were treated with trastuzumab or TDM1(36). CNS penetration by either antibody was equivalent when measured directly by Western blot or immunofluorescence or by inhibition of downstream targets (pAKT, pS6, pERK). In contrast, tumor growth inhibition was greater with TDM1 than trastuzumab suggesting that neither lack of drug penetration nor inadequate CNS concentration was sufficient to explain the differential tumor sensitivity(36).
Although data for HER2-directed small molecule inhibitors is sparse, positron emission tomography (PET) studies in patients with HER2-positive BCBMs using radiolabeled [11C]lapatinib have shown that uptake was seen in brain metastases but not in normal brain tissue(37,38). In addition, CDK4/6 inhibitors, which have shown pre-clinical activity to counteract HER2 resistance mediated by increased cyclin D1 expression in both patient-derived xenograft and transgenic mouse models of non-CNS tumors, can penetrate the brain in mouse models and are being tested in clinical trials(1,39).
Together, data from radiolabeled imaging and clinical response in patients, coupled with antibody binding analysis and downstream target inhibition in orthotopic BCBMs, strongly support HER2 antibody penetration across the BTB. Therefore, if we can eliminate ‘wrong drug’ and demote ‘wrong place’ as reasons for drug resistance, we now need to address the likelihood that we are tackling the ‘wrong target’ in the attempt to kill BCBM tumor cells.
Brain-specific drug resistance mechanisms and tumor adaptations
A growing body of evidence suggests that activation of the PI3K/AKT/mTOR pathway may represent a novel, site-specific, resistance mechanism underlying the poor response of brain metastases to HER2-directed therapy(17,40). Genomic alterations in PI3K/AKT/mTOR pathway have been found in up to 54.5% of breast cancer brain metastases(41). Downstream PI3K pathway activation from loss of PTEN protein expression is also a recurring feature of BCBMs(15,42). Intriguingly, activation of the PI3K pathway, a common genomic hallmark of BCBMs, may be specified by the brain microenvironment. Zhang and colleagues used cell line and conditional knock-out xenograft data to show that PTEN mRNA and protein expression was reduced by CD63+ exosome-delivered microRNA (miR-19a) from tumor-associated astrocytes (right panel, Figure 1)(43).
Breast cancer brain metastases resident within the neural microenvironment also undergo additional brain-mediated modifications that appear to support tumor growth and survival. A comparison of HER2-positive and triple-negative BCBMs to matched primary tumor tissue found increased expression of gamma-amino butyric acid (GABA) transporters in BCBMs, which facilitated tumor use of GABA as a growth-promoting metabolite(44). This hypothesis was supported by the finding that inhibition of GABA transaminase (ABAT) (which converts GABA into succinate and generates NADH as a tricarboxylic acid cycle intermediate) inhibited growth of patient-derived BCBM cells in culture. A related study found that the expression of neural microenvironment-derived extracellular glycoprotein, reelin (which guides neuronal migration and synaptic plasticity in normal neurons), was increased in HER2-positive BCBM and primary tissue(45). In addition, co-culture with BCBM-exposed astrocytes appeared to confer a growth advantage to HER2-positive BCBM cells that was abolished by reelin knock down via shRNA compared to control(45). The increased expression of reelin and its association with astrocyte-mediated growth advantage appeared to be specific to HER2-positive BCBM patient-derived cell lines and HER2-positive cell lines and was not seen triple-negative BCBM patient-derived cell lines. In early postnatal brain development, reelin signals via the PI3K-AKT-mTOR pathway to activate protein translation but whether this is relevant in BCBMs is unknown(46). Together, these data provide additional examples of tumor-specific adaptations may facilitate BCBM survival in the neural microenvironment and may also support drug resistance.
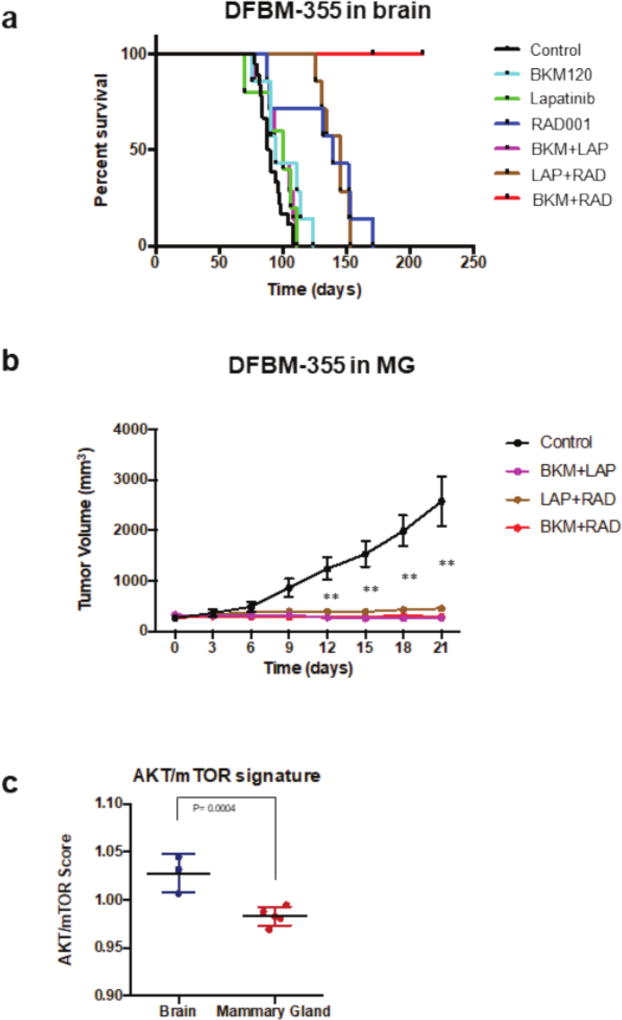
We previously used an orthotopic patient-derived xenograft mouse model of HER2-positive breast cancer brain metastases to demonstrate that susceptibility to dual PI3K+mTOR pathway inhibition was associated with a gene signature of PI3K/AKT/mTOR pathway activation(15). We found that when implanted in the brain of severe combined immunodeficient (SCID) mice, a PTEN-null, patient-derived BCBM xenograft, DFBM-355, responded only to dual PI3K/mTOR inhibition with the combination of BKM120 (Buparlisib) and RAD001 (Everolimus) (Figure 2a). However, when implanted in the mammary gland (MG) all inhibitor combinations (anti-HER2/PI3K, anti-HER2/mTOR, or anti-PI3K/mTOR) inhibited DFBM-355 mammary tumor growth with similar efficacy (Figure 2b). Orthotopic BCBMs (e.g. DFBM-355) sensitive to dual PI3K/mTOR inhibition were associated with increased expression of an AKT-mTOR-dependent gene signature(15). Intriguingly, implanting the identical BCBM, DFBM-355, in the MG vs. brain of SCID mice, resulted in reduction of the AKT/mTOR gene signature (despite remaining PTEN null) and loss of exquisite sensitivity to dual PI3K+mTOR inhibition (Figures 2b, 2c). These data provide further evidence that drug resistance in HER2-positive BCBMs is driven by context-specific activation of PI3K/AKT/mTOR pathway, not failure of drug delivery, and can be inhibited by combination targeted therapy.
Figure 2. HER2-positive BCBM (DFBM-355) has differential drug sensitivity when implanted in the brain compared to mammary gland of SCID mouse.
A) Kaplain-Meier survival curves of DF-BM355/-bearing mice show improved survival by treatment with dual PI3K + mTOR inhibition [BKM120 (30 mg/kg, PO) + RAD001 (7.5 mg/kg, QD)] but not with single agent PI3K, HER2 (lapatinib, 100 mg/kg PO) or mTOR drug inhibition (n=5–18). Adapted from Ni et al, Nat Med 2016. B) When implanted in the mammary gland (MG), DFBM-355 tumor growth inhibition by PI3K+mTOR (BKM+RAD) is equivalent to PI3K+HER2 (BKM+LAP) or HER2+mTOR (LAP+RAD) inhibition. Data shown as mean ± SEM, (n=6–8). Difference tested by two-way ANOVA, followed by Dunnett’s multiple comparison test ** P < 0.01. C) AKT/mTOR gene signature expression is reduced in DFBM-355 implanted in mammary gland compared to the brain. Data are represented as mean ± SD (n = 3–5 per group; difference tested by t-test. P = 0.0004).
In support of these findings, Kodack and colleagues found that cell-line models of BCBMs were resistant to HER2 directed therapy (trastuzumab or lapatinib) when implanted in the brain of immunosuppressed mice but sensitive when implanted in the mammary fat pad(47). They went on to show that HER2-amplified breast cancer cell lines (e.g. BT474) implanted in the brain had increased HER3 expression compared to identical lines implanted in the mammary fat pad and these findings were corroborated in tissue from patients with HER2-positive BCBMs. Intriguingly, they found that resistance to PI3K inhibition (using BKM120, buparlisib) was mediated by increased HER3 activation via expression of the HER3 ligands, neuregulin-1/2, in tumor and stromal cells(16) (right panel, Figure 1). While HER3 inhibition (using the drug LJM 716, which locks HER3 in an inactive conformation) alone did not inhibit tumor growth in orthotopic cell-line BCBM mouse models, combination LJM716 + trastuzumab or buparlisib resistance improved mouse survival compared to control or single agent (trasutuzmab or buparlisib) alone(16) (right panel, Figure 1).
Together, these data suggest that HER2-positive BCBM drug resistance to PI3K or HER2 inhibition occurs from activation at multiple nodes of the PI3K/AKT/mTOR pathway. While single-target inhibition (PI3K, HER2 or HER3) appears ineffective, combining drugs that target both upstream and downstream molecular targets (e.g. HER3 + PI3K or PI3K + mTOR) appears to retard BCBM growth in these models (right panel, Figure 1)(15,16).
In addition to patients with breast cancer, brain metastases most commonly affect patients with melanoma and lung cancer(48). Brain metastases affect approximately 50% of patients with non-small cell lung cancer (NSCLC)(49). Like patients with metastatic HER2-positive breast cancer, patients with metastatic EGFR-mutant (EGFR-m) or ALK-rearranged NSCLC often have intracranial disease progression despite extracranial disease control, a phenomenon that has also been widely attributed to inadequate drug penetration into the CNS(49,50). While the radiolabeled EGFR-inhibitor [11C] erlotinib can be detected by PET in the brains of patients with EGFR-m NSCLC, serum/CSF studies from patients show variable CNS penetration of erlotinib and other tyrosine-kinase inhibitors (TKIs) (e.g. afatinib, and those targeting ALK-rearrangement: crizotinib, ceritinib, alectinib)(49,51,52). Like BCBMs, NSCLC brain metastases also appear to acquire CNS-specific mechanisms of drug resistance. One mechanism of resistance to ALK TKIs, crizotinib and ceritinib, at intracranial sites in mouse models (confirmed in patient sample correlates) is tumor over expression of p-glycoprotein, a drug-efflux transporter(53,54). Like HER2-positive BCBMs, NSCLC brain metastases from patients also acquire brain-specific genomic alterations, for example, in the PI3K-AKT-mTOR pathway, which may contribute to tumor survival and drug resistance(55). Thus, many of the principles used to identify and tackle brain-specific drug resistance mechanisms identified in HER2-positive BCBMs may also be relevant to improving treatments for NSCLC brain metastases.
Brain metastases eventually affect 37% of patients presenting with de novo metastatic melanoma(56). While treatment with BRAF-inhibitor or PD1/L1 inhibitor or CTLA4 inhibitor are not associated with increased risk of developing brain metastasis while on treatment, the median overall survival after a diagnosis of melanoma brain metastasis is only 10.5 months(57). As with HER2-positive breast cancer, brain-specific pathway alterations in melanoma brain metastases may underlie resistance to targeted therapies. In a comparison of 9 metastatic melanoma patients with matched intracranial and extracranial metastases, the incidence of BRAF, NRAS and KIT mutations was the same between both sites(58). However, loss of PTEN expression and increase of phosphorylated-AKT expression by IHC was greater in the intracranial metastases, suggesting preferential activation of the PI3K-AKT pathway in intracranial melanoma metastases, like that seen in HER2-positive BCBMs(15,58). The authors also showed that astrocyte-conditioned media (compared to fibroblast-conditioned media) increased AKT activation in melanoma cells from both intracranial and matched extracranial metastases, suggesting that PI3K-AKT pathway activation seen in patient melanoma brain metastases was the result of brain-specific microenvironmental cues(58). Finally, the same authors also described the clinical history of 7/9 patients where intracranial disease progression of BRAF V600E melanomas occurred on vemurafenib, despite extracranial disease remission, suggesting that a brain-specific drug resistance mechanism was responsible(58). Other studies have also found that PI3K/AKT pathway activation is more frequent in melanoma brain metastases than matched extracranial controls and in case reports of patients with BRAF mutant melanoma, activating mutations of PIK3CA have been associated with drug resistance(59,60). Promisingly, treatment of BRAF-mutant melanoma cell line orthotopic brain metastases in nude mice with the PI3K inhibitor, buparlisib, inhibited tumor growth compared to sham control(61). Together these data suggest that brain-specific resistance mechanisms in melanoma brain metastases may be analogous to those that have been described in HER2-positive BCBMs.
Implications for clinical and translational research
Discordant extracranial and intracranial drug-sensitivity in HER2-positive breast cancer has been largely attributed to poor CNS penetration of trastuzumab and related HER2-directed therapy(9,10). However, careful examination of pre-clinical and clinical data reveals that resistance of HER2-positive BCBMs, especially resistance to trastuzumab, is not solely due to poor drug delivery across the blood-tumor barrier. Instead, HER2-positive BCBMs are characterized by activation of the PI3K-AKT-mTOR pathway likely via multiple mechanisms, including PTEN loss and HER3 activation, which appear to be driven by molecular cues from the brain microenvironment (Figures 1,2)(15,16,43). In this respect, HER2-positive BCBM recapitulate pathways of adaptive resistance (upregulation of HER3) and acquired resistance (loss of PTEN, activation of AKT-MTOR signaling) that have been previously described in the context of systemic metastases treated with PI3K/mTOR pathway inhibitors(62).These insights suggest an approach for understanding intracranial drug resistance and identifying novel therapeutic strategies that have the potential to improve patient care (Figure 3,4).
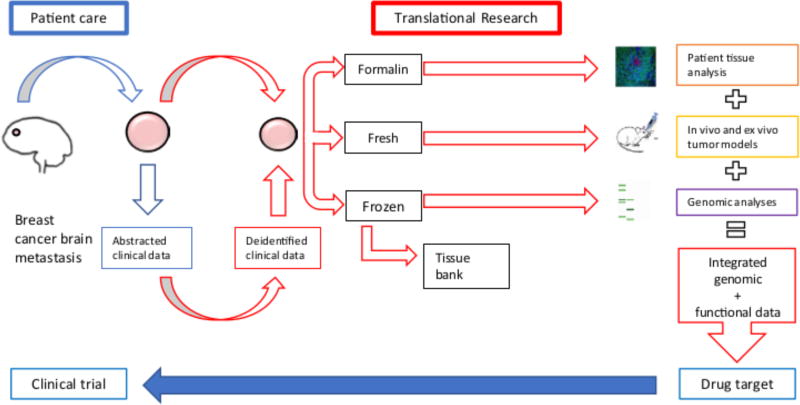
Figure 3. DFCI Breast Cancer Brain Metastasis translational workflow.
This sample workflow adopted at our institution, Dana Farber Cancer Institute (DFCI) allows for rapid and multi-level analysis of genomic, protein and functional changes (translational research) specific to breast cancer brain metastases. Data from descriptive and functional studies is integrated to identify candidate targets for drug testing in clinical trials (patient care). Images reproduced from Ni et al, Nat Med 2016 and NCI Visuals Online of ‘Treatment Resistant Breast Cancer Cells’ https://visualsonline.cancer.gov/details.cfm?imageid=10574
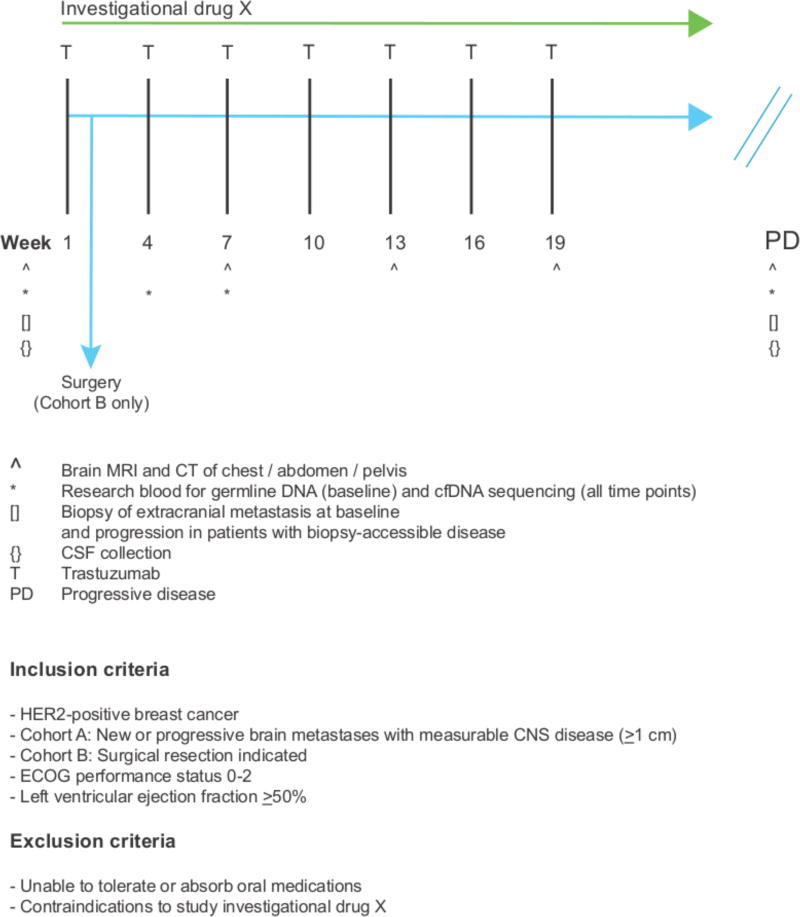
Figure 4. Model clinical trial scheme testing an investigational drug in patients with HER2-positive BCBMs.
A clinical trial testing a rationally chosen drug in patients with HER2-positive BCBMs should consist of two cohorts: Cohort A, an open-label, single-arm, two-stage, phase II cohort: the ‘efficacy cohort’; and, Cohort B, a pre-surgical window cohort: the ‘biomarker evaluation / discovery’ cohort (see text for details). Patients are treated with a trastuzmab (T) backbone in combination with drug X. For Cohort A, the primary endpoint would be CNS objective response rate as measured by RANO-BM criteria. For Cohort B, the primary endpoint would be inhibition of drug-specific pharmacodynamic markers in resected brain tumor tissue. Secondary clinical endpoints will include other pharmacodynamic biomarkers e.g. change in cfDNA with treatment, in addition to clinical outcomes.
Drug resistance in HER2-positive BCBMs appears, in part, to be driven by activation of the PI3K/AKT/mTOR pathway, immediately suggesting potential targeted drug combinations for testing in clinical trials. While the efficacy of PI3K inhibitors has been limited in breast cancer, the use of rational drug combinations, with alternate dosing and non-overlapping side effects, may be a promising strategy for minimizing toxicity while maximizing activity in preclinical studies(62,63).
Analysis of post-treatment tumor tissue to identify mechanisms of resistance and suggest new drug options is a well-established approach that depends on retrieval of primary or treated metastases from patients for study ex vivo or in vitro(64,65). Patient-derived tissue biobanks can greatly facilitate correlative and functional studies to determine the efficacy of novel drug / drug-combinations and identify resistance mechanisms(66). However, while resection of primary brain tumors is standard of care, brain metastases are either biopsied or resected only in cases of diagnostic uncertainty (when no extracranial site is available) or clinical necessity(67). Given these challenges, the use of CSF cell-free DNA (cfDNA) (with matched serum cfDNA as extracranial control) offers an alternative method of identifying potentially actionable mutations or copy number changes in BCBMs that merits validation in clinical trials(68).
Because BCBM drug resistance can arise from molecular cross-talk between components of the brain microenvironment and metastatic cancer cells, pre-clinical target validation and testing in orthotopic tumor rodent models should be incorporated as a critical step in the drug development process(16). While rodent xenograft models are widely used to representing the complexity inherent in BCBM-stroma-neurovascular unit interactions, they have limitations—not least being the absence of a functional host immune system. Use of syngeneic rodent tumor models and ex vivo systems such as organotypic slice culture could overcome some of these limitations by allowing drug testing within an immunocompetent microenvironment(69).
The heterogeneous and unpredictable drug permeability of blood-tumor barrier remains a challenge to designing effective therapies for BCBMs, notwithstanding co-existing drug resistance mechanisms(25). Microfluidic co-culture models, intravital microscopy or fluorescence deep-tissue and non-invasive imaging of orthotopic models in conjunction with tagged-nanoparticle delivery systems can all help advance our understanding of the determinants of BTB permeability and suggest methods of targeted drug-delivery across the BTB(70–72).
The prevalent concern that most drugs are not likely to penetrate the BTB results in patients with brain metastases from breast cancer (and other tumor sites) being excluded from clinical trials(73). This reduces the exposure of patients with brain metastases to potentially-beneficial novel agents as well as limiting importantly correlative science around brain-specific tumor responses that is necessary to spur the development of CNS-effective drugs. An American Society of Clinical Oncology/Friends of Cancer Research Brain Metastases working group has made increasing brain metastasis patients clinical trials enrolment a top priority and a report detailing the scope of the problem and suggested recommendations has been recently published(74). Our hope is that by dispelling the automatic assumption that intracranial and extracranial drug efficacy are simply related to drug penetrance, we will stimulate the design, execution and enrollment of clinical trials for patients with brain metastases from breast cancer and other tumor sites.
To that end, Figure 4 shows an example of clinical trial design that aims to test the CNS activity of investigational drug X in patients with HER2-positive BCBMs. As discussed above, drug activity against BCBMs may be limited by CNS-specific resistance mechanisms or failure of drug delivery. Therefore, confirming an investigational drug’s on-target CNS activity and potential CNS-specific resistance mechanisms should be an important co-objective of any clinical trial and requires trial design that facilitates the acquisition of pre-/post-treatment tumor tissue. We propose that a clinical trial of an investigational drug in patients with HER2-positive BCBMs should include a modestly-sized ‘biomarker evaluation/discovery’ cohort (for example, with a pre-surgical window cohort, where patients are treated with investigational agents prior to surgical resection of a brain metastasis: Cohort B, Figure 4) in parallel with a primary ‘efficacy cohort’ (Cohort A, Figure 4). For patients with accessible extracranial disease in both cohorts, biopsies before and after investigational drug exposure could be collected to compare intracranial vs. extracranial drug response and resistance mechanisms. Given the need to develop less invasive methods for biomarker evaluation, serial cfDNA from plasma and CSF could also be collected, although such assays provide limited information about the contribution of the tumor microenvironment to drug response. The primary endpoint of the efficacy cohort could be CNS objective response rate as measured by the RANO-BM criteria(75). For the pre-surgical window cohort, several primary endpoints would be possible, including degree of pathway suppression, or even correlation of biomarker patterns with response in PDX models generated from the same surgically collected samples. Indeed, generation of PDX models from patients in the ‘biomarker/discovery’ cohort (as in Figure 3) could also provide the ability to test the efficacy of compounds in tumor engrafted into varying sites (e.g. brain vs lung vs mammary fat pad) and then to correlate these with clinical outcomes at intracranial vs extracranial sites in the same patient treated on the trial.
Conclusions
Despite decades of progress in treating metastatic breast cancer with systemic drugs, HER2-positive breast cancer brain metastases still connote a guarded prognosis(1). Even more challenging for patient and treating clinician is intracranial disease progression on HER2-directed therapy despite extracranial disease control. This problem has been largely attributed to poor penetration of chemotherapy and targeted therapies across the blood-tumor barrier. In fact, evidence from experimental models and patients show that tumor drug resistance occurs despite adequate delivery of small molecules and monoclonal antibodies across the BTB. Orthotopic BCBM xenograft experiments have shown that the PI3K-AKT-mTOR pathway is frequently activated as a brain-specific mechanism of drug resistance to HER2-targeted therapies but may be overcome using combination therapy e.g. PI3K + mTOR inhibitors. These data suggest that we cannot predict intracranial drug efficacy by simple extrapolation from extracranial drug efficacy data, even if a drug were to cross the BTB perfectly. However, a research strategy that synergizes the complementary skill sets of laboratory scientists and clinical investigators using patient-derived BCBM tumor tissue in orthotopic models to identify context-specific resistance mechanisms may identify drug targets that could be tested in clinical trials to successfully treat HER2-positive BCBMs.
Statement of translational relevance.
Patients with active breast cancer brain metastases (BCBMs) are often excluded from clinical trials because of the concern that most drugs do not adequately penetrate the blood-tumor barrier. This impedes both discovery and validation of drug targets for treating BCBMs. We reviewed pharmacodynamic data from HER2-positive breast cancer brain metastasis mouse models and patients, which suggest that BCBMs resist HER2-targeted therapy despite adequate intracranial drug delivery and activity. In fact, evidence suggests that brain-specific molecular alterations involving the PI3K-AKT-mTOR pathway underlie BCBM resistance to HER2-targeted therapy. Thus, careful integration of data from in vivo models, non-invasive imaging and patient tissues can better determine drug activity at metastatic sites and help reveal novel resistance mechanisms. By highlighting drug resistance in brain metastases as a tractable problem, we aim to stimulate further basic and translational investigation in this underserved research field.
Acknowledgments
We gratefully acknowledge the work of Victor Luu and Yanzhi Wang in performing the animal experiments shown in this manuscript.
We gratefully acknowledge following organizations for funding and supporting this work:
National Cancer Institute, Specialized Program of Research Excellence (SPORE) Grant 1P50CA168504 (E Winer, N Lin, J Zhao, S Kabraji, J Ning, S Xie).
National Cancer Institute, Outstanding Investigator Award R35 CA210057 (J Zhao)
Breast Cancer Research Foundation (N Lin and E Winer)
Breast Cancer Research Foundation (J Zhao)
List of Abbreviations
- BCBM
Breast cancer brain metastases
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
References
- 1.Lin NU, Gaspar LE, Soffietti R. Breast Cancer in the Central Nervous System: Multidisciplinary Considerations and Management. Am Soc Clin Oncol Educ Book. 2017;37:45–56. doi: 10.14694/EDBK_175338. [DOI] [PubMed] [Google Scholar]
- 2.Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112(3):467–72. doi: 10.1007/s11060-013-1083-9. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2100–8. doi: 10.1200/JCO.2013.54.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin NU. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 2013;7:307. doi: 10.3332/ecancer.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin NU. Brain metastases in HER2-positive breast cancer. Lancet Oncol. 2013;14(3):185–6. doi: 10.1016/S1470-2045(13)70046-9. [DOI] [PubMed] [Google Scholar]
- 7.Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19(23):6404–18. doi: 10.1158/1078-0432.CCR-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14(3):244–8. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 9.Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24(6):1526–33. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 11.Larsen PB, Kumler I, Nielsen DL. A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat Rev. 2013;39(7):720–7. doi: 10.1016/j.ctrv.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ. The Biology of Brain Metastasis: Challenges for Therapy. Cancer J. 2015;21(4):284–93. doi: 10.1097/PPO.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 13.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 15.Ni J, Ramkissoon SH, Xie S, Goel S, Stover DG, Guo H, et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016;22(7):723–6. doi: 10.1038/nm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, Goel S, et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2017;9(391) doi: 10.1126/scitranslmed.aal4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagogo-Jack I, Gill CM, Cahill DP, Santagata S, Brastianos PK. Treatment of brain metastases in the modern genomic era. Pharmacol Ther. 2017;170:64–72. doi: 10.1016/j.pharmthera.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Lin NU. Better treatments needed for breast cancer brain metastases. Lancet Oncol. 2015;16(16):1583–4. doi: 10.1016/S1470-2045(15)00477-5. [DOI] [PubMed] [Google Scholar]
- 19.Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28(8):1301–7. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 20.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–77. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 21.Freedman RA, Gelman RS, Wefel JS, Melisko ME, Hess KR, Connolly RM, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: A Phase II Trial of Neratinib for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol. 2016;34(9):945–52. doi: 10.1200/JCO.2015.63.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittapalli RK, Adkins CE, Bohn KA, Mohammad AS, Lockman JA, Lockman PR. Quantitative Fluorescence Microscopy Measures Vascular Pore Size in Primary and Metastatic Brain Tumors. Cancer Res. 2017;77(2):238–46. doi: 10.1158/0008-5472.CAN-16-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegram MD. Neratinib in ERBB2-Positive Brain Metastases. JAMA Oncol. 2016;2(12):1541–3. doi: 10.1001/jamaoncol.2016.0238. [DOI] [PubMed] [Google Scholar]
- 24.Freedman RA, Gelman RS, Melisko ME, Anders CK, Moy B, Blackwell KL, et al. TBCRC 022: Phase II trial of neratinib + capecitabine for patients (Pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer brain metastases (BCBM) Journal of Clinical Oncology. 2017;35(15_suppl):1005. doi: 10.1200/JCO.2017.35.15_suppl.1005. [DOI] [Google Scholar]
- 25.Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 2017;31(3):326–41. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–82. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohn JP, Pall G, Stockhammer G, Steurer M. Targeted Therapies for the Treatment of Brain Metastases in Solid Tumors. Target Oncol. 2016;11(3):263–75. doi: 10.1007/s11523-015-0414-5. [DOI] [PubMed] [Google Scholar]
- 29.Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E, et al. Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin Cancer Res. 2016;22(21):5287–99. doi: 10.1158/1078-0432.CCR-15-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massague J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 2017;168(6):1101–13. e13. doi: 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doolittle ND, Peereboom DM, Christoforidis GA, Hall WA, Palmieri D, Brock PR, et al. Delivery of chemotherapy and antibodies across the blood-brain barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the Eleventh Annual Blood-Brain Barrier Consortium meeting. J Neurooncol. 2007;81(1):81–91. doi: 10.1007/s11060-006-9209-y. [DOI] [PubMed] [Google Scholar]
- 33.Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15(2):219–25. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Lewis Phillips GD, Nishimura MC, Lacap JA, Kharbanda S, Mai E, Tien J, et al. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res Treat. 2017;164(3):581–91. doi: 10.1007/s10549-017-4279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54(11):1869–75. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 36.Askoxylakis V, Ferraro GB, Kodack DP, Badeaux M, Shankaraiah RC, Seano G, et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microenvironment. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleem A, Searle GE, Kenny LM, Huiban M, Kozlowski K, Waldman AD, et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 2015;5:30. doi: 10.1186/s13550-015-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morikawa A, Peereboom DM, Thorsheim HR, Samala R, Balyan R, Murphy CG, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2015;17(2):289–95. doi: 10.1093/neuonc/nou141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, et al. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab Dispos. 2015;43(9):1360–71. doi: 10.1124/dmd.114.062745. [DOI] [PubMed] [Google Scholar]
- 40.Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK. Emerging strategies for treating brain metastases from breast cancer. Cancer Cell. 2015;27(2):163–75. doi: 10.1016/j.ccell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunus JM, Quinn MC, Patch AM, Pearson JV, Bailey PJ, Nones K, et al. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. 2015;237(3):363–78. doi: 10.1002/path.4583. [DOI] [PubMed] [Google Scholar]
- 42.Wikman H, Lamszus K, Detels N, Uslar L, Wrage M, Benner C, et al. Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Res. 2012;14(2):R49. doi: 10.1186/bcr3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci U S A. 2014;111(3):984–9. doi: 10.1073/pnas.1322098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jandial R, Choy C, Levy DM, Chen MY, Ansari KI. Astrocyte-induced Reelin expression drives proliferation of Her2(+) breast cancer metastases. Clin Exp Metastasis. 2017;34(2):185–96. doi: 10.1007/s10585-017-9839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee GH, D'Arcangelo G. New Insights into Reelin-Mediated Signaling Pathways. Front Cell Neurosci. 2016;10:122. doi: 10.3389/fncel.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci U S A. 2012;109(45):E3119–27. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQ. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol. 2017;19(1):i1–i24. doi: 10.1093/neuonc/now197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baik CS, Chamberlain MC, Chow LQ. Targeted Therapy for Brain Metastases in EGFR-Mutated and ALK-Rearranged Non-Small-Cell Lung Cancer. J Thorac Oncol. 2015;10(9):1268–78. doi: 10.1097/JTO.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 50.Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol. 2015;33(17):1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 52.Weber B, Winterdahl M, Memon A, Sorensen BS, Keiding S, Sorensen L, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol. 2011;6(7):1287–9. doi: 10.1097/JTO.0b013e318219ab87. [DOI] [PubMed] [Google Scholar]
- 53.Kort A, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) Pharmacol Res. 2015;102:200–7. doi: 10.1016/j.phrs.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, et al. P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine. 2016;3:54–66. doi: 10.1016/j.ebiom.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015;5(11):1164–77. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 57.Sloot S, Chen YA, Zhao X, Weber JL, Benedict JJ, Mule JJ, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer. 2017 doi: 10.1002/cncr.30946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013;2(1):76–85. doi: 10.1002/cam4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harding JJ, Catalanotti F, Munhoz RR, Cheng DT, Yaqubie A, Kelly N, et al. A Retrospective Evaluation of Vemurafenib as Treatment for BRAF-Mutant Melanoma Brain Metastases. Oncologist. 2015;20(7):789–97. doi: 10.1634/theoncologist.2014-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen M, Nowak DG, Trotman LC. Molecular pathways: PI3K pathway phosphatases as biomarkers for cancer prognosis and therapy. Clin Cancer Res. 2014;20(12):3057–63. doi: 10.1158/1078-0432.CCR-12-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niessner H, Schmitz J, Tabatabai G, Schmid AM, Calaminus C, Sinnberg T, et al. PI3K Pathway Inhibition Achieves Potent Antitumor Activity in Melanoma Brain Metastases In Vitro and In Vivo. Clin Cancer Res. 2016;22(23):5818–28. doi: 10.1158/1078-0432.CCR-16-0064. [DOI] [PubMed] [Google Scholar]
- 62.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017;170(4):605–35. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue Y, Martelotto L, Baslan T, Vides A, Solomon M, Mai TT, et al. An approach to suppress the evolution of resistance in BRAFV600E-mutant cancer. Nat Med. 2017;23(8):929–37. doi: 10.1038/nm.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, et al. Circulating Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-17-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518(7538):240–4. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bins S, Cirkel GA, Gadellaa-Van Hooijdonk CG, Weeber F, Numan IJ, Bruggink AH, et al. Implementation of a Multicenter Biobanking Collaboration for Next-Generation Sequencing-Based Biomarker Discovery Based on Fresh Frozen Pretreatment Tumor Tissue Biopsies. Oncologist. 2017;22(1):33–40. doi: 10.1634/theoncologist.2016-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nabors LB, Portnow J, Ammirati M, Brem H, Brown P, Butowski N, et al. Central nervous system cancers, version 2.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2014;12(11):1517–23. doi: 10.6004/jnccn.2014.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Mattos-Arruda L, Mayor R, Ng CK, Weigelt B, Martinez-Ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. doi: 10.1038/ncomms9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chadwick EJ, Yang DP, Filbin MG, Mazzola E, Sun Y, Behar O, et al. A Brain Tumor/Organotypic Slice Co-culture System for Studying Tumor Microenvironment and Targeted Drug Therapies. J Vis Exp. 2015;105:e53304. doi: 10.3791/53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tosi U, Marnell CS, Chang R, Cho WC, Ting R, Maachani UB, et al. Advances in Molecular Imaging of Locally Delivered Targeted Therapeutics for Central Nervous System Tumors. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lagerweij T, Dusoswa SA, Negrean A, Hendrikx EML, de Vries HE, Kole J, et al. Optical clearing and fluorescence deep-tissue imaging for 3D quantitative analysis of the brain tumor microenvironment. Angiogenesis. 2017 doi: 10.1007/s10456-017-9565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terrell-Hall TB, Ammer AG, Griffith JI, Lockman PR. Permeability across a novel microfluidic blood-tumor barrier model. Fluids Barriers CNS. 2017;14(1):3. doi: 10.1186/s12987-017-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costa R, Gill N, Rademaker AW, Carneiro BA, Chae YK, Kumthekar P, et al. Systematic analysis of early phase clinical studies for patients with breast cancer: Inclusion of patients with brain metastasis. Cancer Treat Rev. 2017;55:10–5. doi: 10.1016/j.ctrv.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Lin NU, Prowell T, Tan AR, Kozak M, Rosen O, Amiri-Kordestani L, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017 doi: 10.1200/JCO.2017.74.0761. JCO2017740761. [DOI] [PubMed] [Google Scholar]
- 75.Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–8. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]