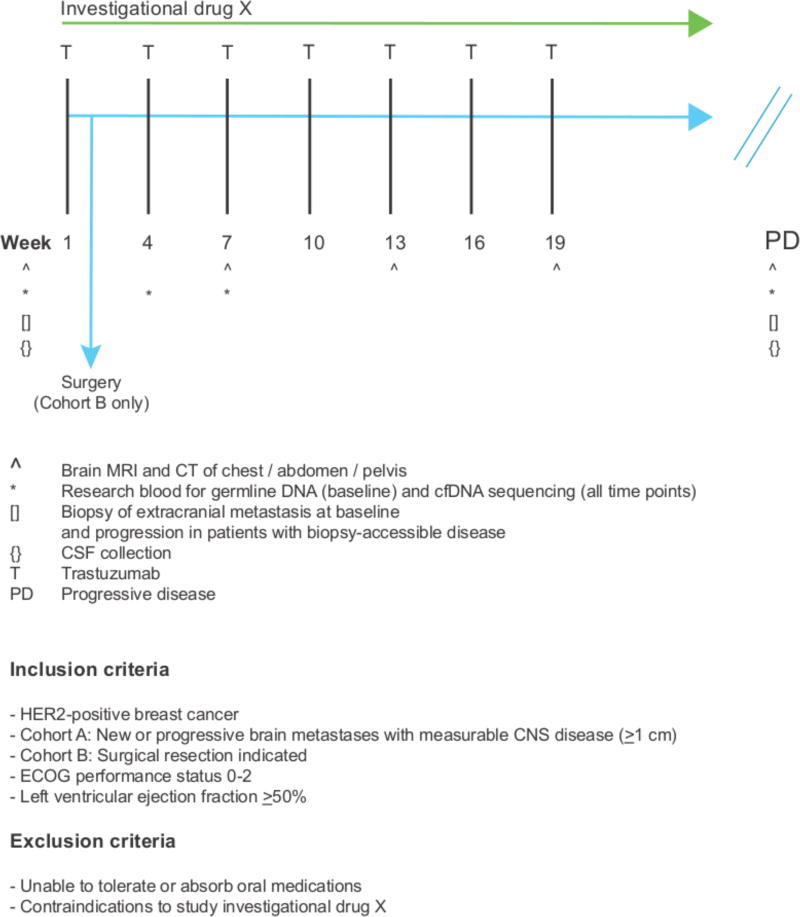
Figure 4. Model clinical trial scheme testing an investigational drug in patients with HER2-positive BCBMs.
A clinical trial testing a rationally chosen drug in patients with HER2-positive BCBMs should consist of two cohorts: Cohort A, an open-label, single-arm, two-stage, phase II cohort: the ‘efficacy cohort’; and, Cohort B, a pre-surgical window cohort: the ‘biomarker evaluation / discovery’ cohort (see text for details). Patients are treated with a trastuzmab (T) backbone in combination with drug X. For Cohort A, the primary endpoint would be CNS objective response rate as measured by RANO-BM criteria. For Cohort B, the primary endpoint would be inhibition of drug-specific pharmacodynamic markers in resected brain tumor tissue. Secondary clinical endpoints will include other pharmacodynamic biomarkers e.g. change in cfDNA with treatment, in addition to clinical outcomes.