Figure 1.
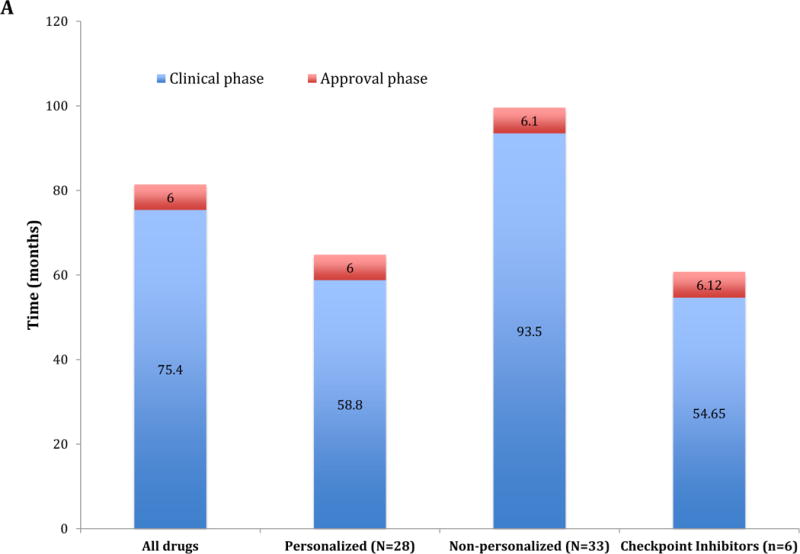
A: Comparison of the drug development timeline (Clinical and Approval phase) between 61 anticancer drugs approved by the FDA between 09/1999 and 07/2014 and immune checkpoint inhibitors approved until 06/2017. The 61 drugs were also stratified under personalized (n = 28 drugs) and non-personalized (n = 33 drugs) according to the development strategy. “Personalized” indicates biomarker-based approval.
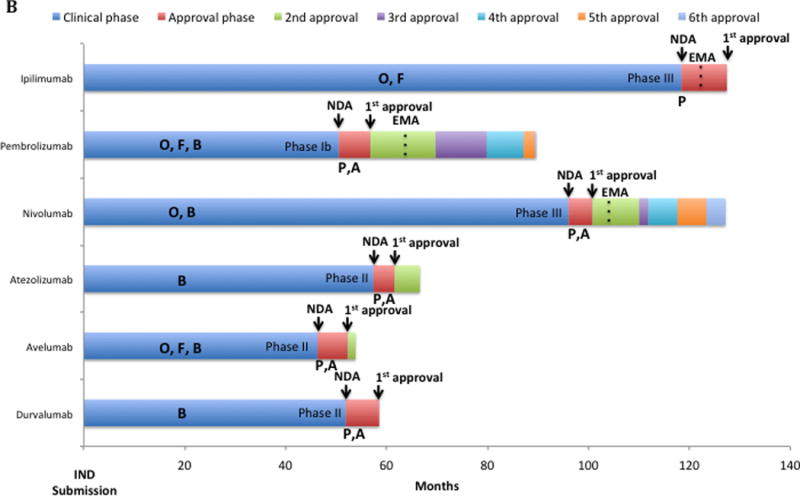
B: Drug development Timeline of Immune Checkpoints inhibitors approved by the FDA. Each bar represents in months the time for each step of clinical development. Letters inside clinical phase and below approval phase represent access to each FDA-expedited program. At the end of blue bars the type of registration trial submitted for first approval is described (e.g., Phase I, Phase II or Phase III trial). Dashed lines inside bars are the moment in time of EMA approval (if received). Time from first FDA to first EMA approval: ipilimumab, 3.6 months; pembrolizumab, 10.4 months; nivolumab, 5.9 months. Clinical phase is time from IND submission to the FDA (necessary before first-in-human trial initiates) to the submission for new drug application (NDA). Approval phase is the time between the NDA submission to the FDA and the approval by the FDA of the drug for marketing.
Abbreviations: A: accelerated approval; B: breakthrough designation; EMA: European Medical Agency; F: fast-track: IND: investigational new drug; NDA: new drug application; O: orphan drug status; P: priority review
Definitions for these types of special FDA designations are given in Supplemental Table 3.
