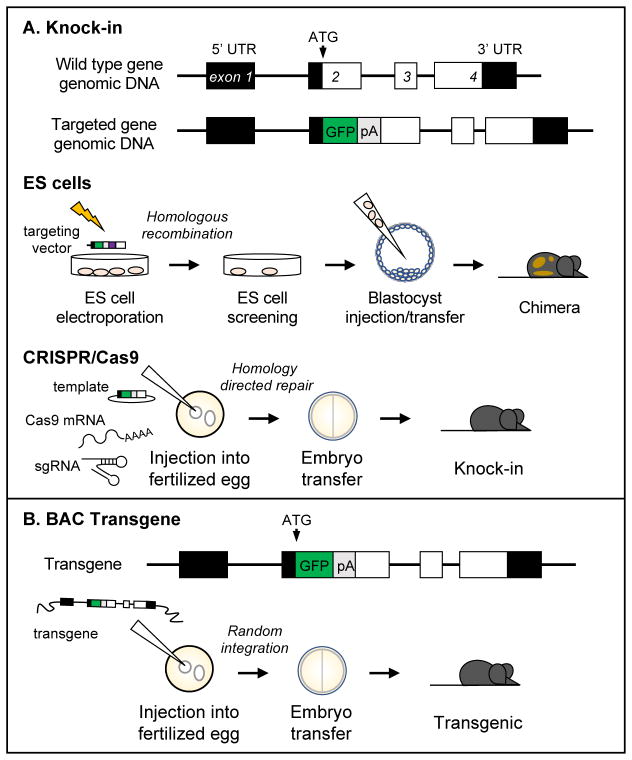
Figure 1. Use of KI and Tg approaches to generate HSC-specific genetic reporter animals.
Both strategies use regulatory elements of a gene of interest to drive expression of a reporter cassette. This cassette can be inserted 5′ (as depicted above, immediately after the ATG start codon) or 3′ of the coding sequence. (A) KI animals can be generated by targeting of ES cells or using the CRISPR/Cas9 system. To modify ES cells, a targeting vector that encodes reporter and antibiotic resistance cassettes flanked by arms of homologous sequence both directly 5′ and 3′ of the targeted locus is electroporated into ES cells. These flanking arms facilitate homologous recombination between the vector insert and ES cell genomic DNA, and ES cells are selected for the appropriate antibiotic resistance and screened for targeting vector incorporation. Correctly targeted ES cells are injected into a blastocyst of a different genetic background, which is transferred to a pseudopregnant female. ES cell contribution to the resulting pup is visualized by the different coat colors associated with the genetic backgrounds of the ES cells and blastocyst. Chimeric animals must be further bred to confirm germline contribution from the ES cells. Alternatively, KI animals can be generated by the direct injection of mRNA encoding the Cas9 nuclease, an sgRNA that directs DNA binding of Cas9, and a donor template vector encoding the reporter cassette into a fertilized egg. DSB mediated by Cas9 can be resolved by HDR, leading to incorporation of the reporter cassette at the desired genomic locus. The zygote is transferred to a pseudopregnant female, and the resulting progeny are screened for the correct genotype. (B) BAC Tg animals are made in a similar manner to KI animals made using CRISPR/Cas9. A reporter cassette is introduced into a BAC clone that encodes the locus of interest by recombineering. The targeted BAC DNA becomes randomly incorporated into the genomic DNA of a fertilized egg, which is then transferred to a pseudopregnant female mouse. Resulting offspring are genotyped for the Tg.