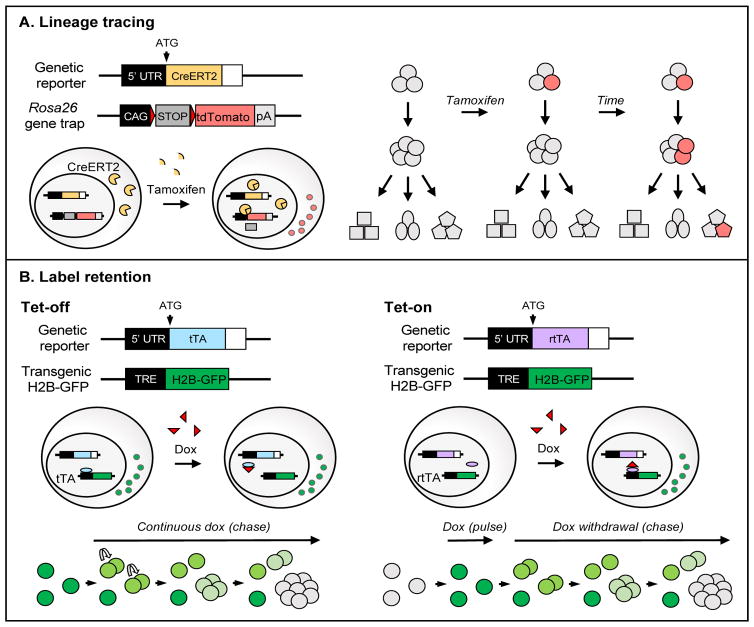
Figure 2. Studying HSC functions in vivo using genetic reporter systems.
HSC-specific reporters can be combined with other inducible genetic mouse strains to study in vivo functions of HSCs. (A) In vivo HSC contribution to hematopoiesis can be studied by lineage tracing. In the absence of tamoxifen, the CreERT2 recombinase is retained in the cytoplasm and a LoxP-flanked Stop cassette encoding repeating polyadenylation signals prevents expression of the fluorescent reporter (e.g. tdTomato) in cells that express the HSC reporter. Tamoxifen treatment enables CreERT2 translocation to the nucleus where it mediates excision of the Stop cassette. This results in irreversible expression of tdTomato, and any daughter cell that arises from the labeled cell will also express tdTomato. The contribution of the labeled cell to the production of other cell types is estimated by measuring the fraction of tdTomato+ cells over time. (B) Dormant HSCs can be identified in vivo by the ability to stably retain human histone H2B-GFP fluorescent label. A TRE regulates the expression of H2B-GFP, and this system can function as a “Tet-off” or “Tet-on” system. In Tet-off systems, H2B-GFP is induced in cells that express the HSC-specific tTA. In the presence of the tetracycline analog doxycycline (dox) the de novo synthesis of H2B-GFP is inhibited. The H2B-GFP protein is highly stable but becomes diluted upon proliferation of labeled cells over time. Cells that retain the fluorescent label over a long period of continuous dox treatment are considered stable label retaining cells. In a Tet-on system, H2B-GFP expression is induced in cells that express the HSC-specific reverse tetracycline transactivator (rtTA) only upon treatment with dox. Once complete labeling of the population of interest is confirmed, dox is removed and cells are assessed for label retention over time.