Figure 4. Vxn is required for primary neuron differentiation.
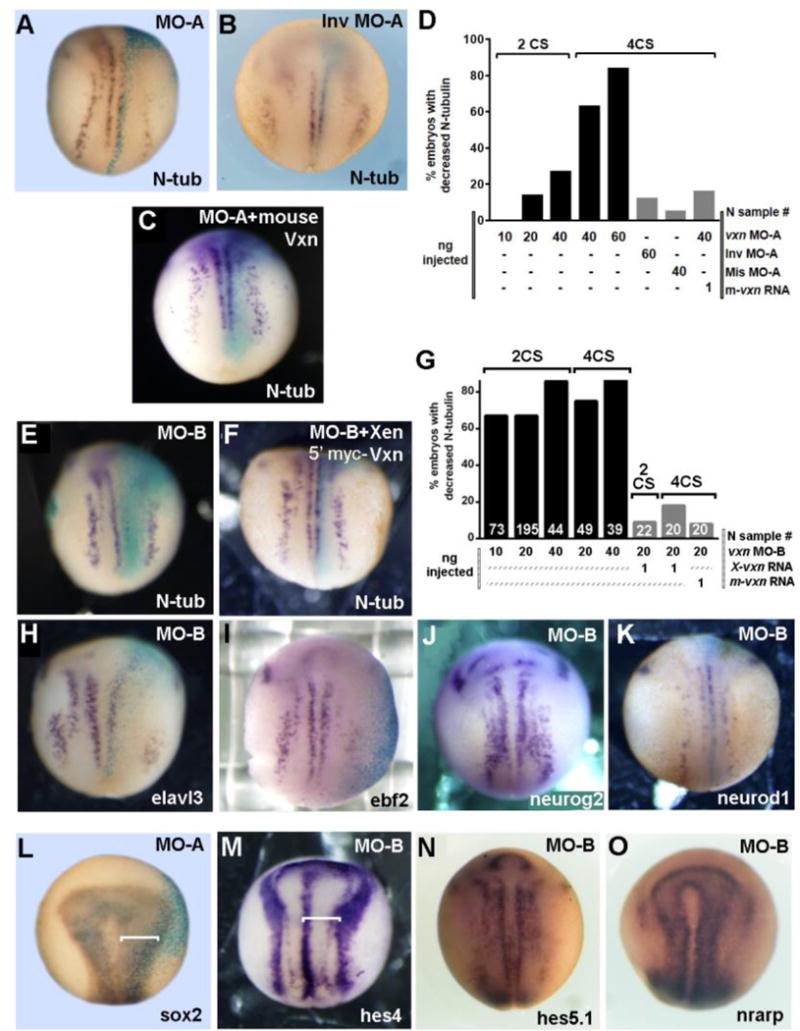
(A-E) Injection 20ng of vxn translation-blocking morpholino (MO-A) at the 4-cell stage (4CS) causes reduced N-tubulin (N-tub) positive primary neurons at stage 14 (A), with increased inhibition at increasing doses of MO-A (D), while injection of 20ng of inverted morpholino (Inv MO-A) does not (B). Primary neurogenesis is rescued by co-injection of vxn MO-A with 1ng of mouse vxn mRNA (C, D).
(E-G) Primary neurogenesis is inhibited by injection of 20ng of MO-B at the 4CS (E), with efficient inhibition with injection of 10-40ng of vxn MO-B at the 2 or 4 CS (G). Loss of N-tub can be rescued by co-injection of 1ng of Xenopus 5′myc-vxn (F) or mouse (G) vxn mRNA. (H-O) Injection of vxn MO-B inhibits expression of other markers of differentiating primary neurons, including elavl3 (H) and ebf2 (I), but has no effects on expression of neurogenic bHLH genes such as neurog2 (J) or neurod1 (K).
(L, M) Injection of vxn MO-A or MO-B causes expansion of progenitors in the neural plate as revealed by expansion of sox2 (L) or the neural plate domain bounded by hes4 (M).
(N, O) Injection of vxn MO-B causes no change in the expression of Notch pathway genes such as hes5.1 (N) and nrarp (O).
For all experiments, mRNA encoding either GFP or β-galactosidase was co-injected to label the injected side (right). X-gal staining is light blue.
