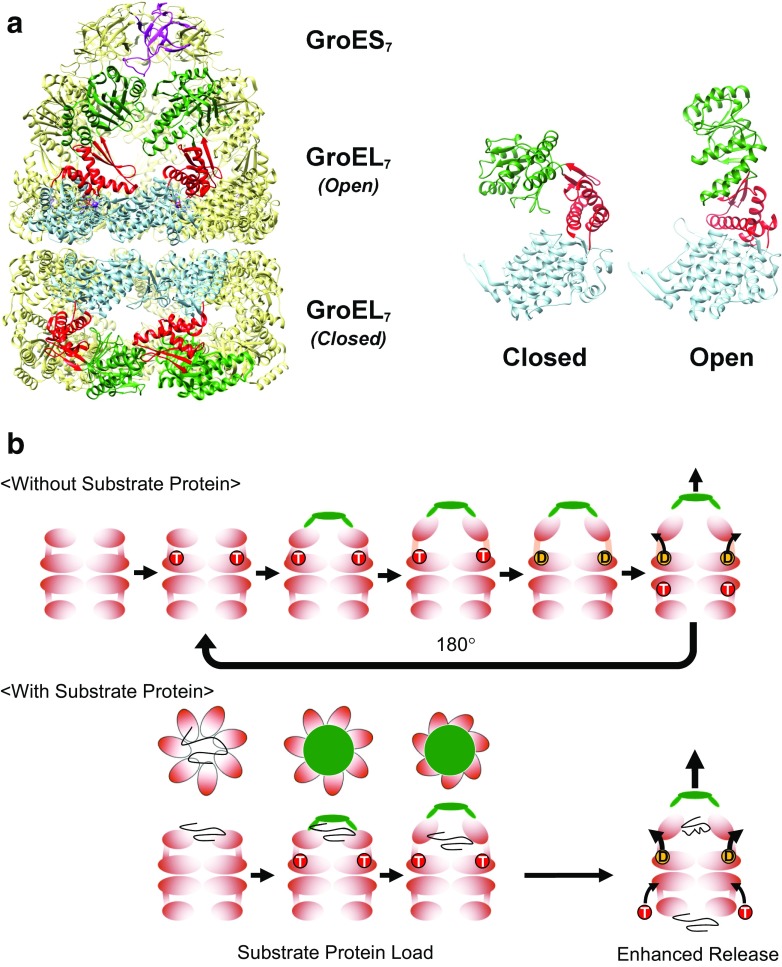
Fig. 1.
The GroE chaperonin system from Escherichia coli. a (Left) The architecture of the GroE system. Fourteen subunits of GroEL form two heptameric rings (GroEL7) that are associated back to back. In the presence of nucleotides such as ATP, the cochaperonin GroES (GroES7) binds to GroEL to form the cis–GroEL–GroES complex depicted in the figure. Four subunits in the foreground of the GroEL 14-mer are colored by domain; the equatorial domain in light blue, the intermediate domain in red, and the apical domain in dark green. One subunit of GroES is colored magenta. (Right) The two GroEL subunits depicted show the two main conformations of GroEL (Closed and Open) that are observable in the crystal structure shown here (1SVT, Chaudhry et al. 2004). Crystal structures in this and subsequent figures were drawn using UCSF Chimera (Pettersen et al. 2004). b (Without Substrate Protein) The basic functional cycle of GroE. Binding and hydrolysis of ATP triggers various conformational changes that trigger GroES binding, formation of the encapsulating complex, and cycling. In the absence of unfolded polypeptide (the substrate protein), allosteric mechanisms cause the cycle to favor an asymmetric form where ATP binding and hydrolysis occur on alternating GroEL heptamers. (With Substrate Protein) The presence of unfolded substrate proteins mainly affects two facets of the basic cycle outlined above; first, the substrate proteins bind multivalently to the apical domains of GroEL to apply a “load” on the molecule, which must be overcome by the conformational changes of GroEL, as well as the binding of GroES (“Substrate Protein Load”). Substrate binding also confers an additional effect where the release of hydrolyzed ADP and bound GroES is enhanced by the binding of unfolded peptide to the trans ring (“Enhanced Release”)