Abstract
Background and Aims
Weight regain after Roux-en-Y gastric bypass (RYGB) correlates with dilated gastrojejunal anastomosis (GJA). Endoscopic sutured transoral outlet reduction (TORe) is safe and effective in the management, and has predominantly been performed by either (1) placing interrupted sutures at the GJA or (2) the creation of a pursestring. The aim of the current study was to compare these techniques.
Methods
All patients undergoing TORe using for weight regain after RYGB were prospectively enrolled. Primary outcome was mean percent total weight loss (%TWL) at 3 and 12 months. Secondary outcomes included percent excesss weight loss (%EWL), percent regained weight lost (%RWL), and total weight loss. Proportions were compared using fisher’s exact test and continuous variables using the student t-test. A p-value of 0.05 was significant. Multivariable regression analysis was performed.
Results
Two hundred forty-one patients were enrolled (pursestring=187, interrupted =54). There was no statistical difference between the pursestring and interrupted group at 3 months in %TWL (8.6 vs. 8.0, p=0.41), %EWL (20.5 vs 16.7, p=0.39), % RWL (44.7 vs 33.3, p=0.56), and total weight loss (9.5 vs 11.3, p=0.32). At 12 months, the pursestring group achieved statistically significant improvement in %TWL (8.6 vs 6.4, p=0.02), %EWL (19.8 vs 11.7, p=<0.001), % RWL (40.2 vs 27.8, p=0.02), and total weight loss (9.5 vs 7.8, p=0.04). Multivariable regression showed technique (p=0.006) was an independent predictor of %TWL at 12 months.
Conclusion
TORe is effective in treatment of weight regain after failed gastric bypass. The pursestring technique results in greater weight loss at 12 months than the traditional interrupted suture pattern.
Keywords: Roux-en-Y, gastric bypass, anastomosis, endoscopy, obesity, weight gain
INTRODUCTION
Obesity is a growing epidemic worldwide, and is associated with increased risk of hypertension, diabetes, heart disease, sleep apnea, and hyperlipidemia1–5. Increasing rates of obesity are predicted to lead to an overall decline in life expectancy in the United States. Bariatric surgery has proved to be effective in the treatment of obesity, leading to sustained weight loss and the improvement of medical comorbidities2.
Roux-en-Y gastric bypass (RYGB) is a commonly performed bariatric surgeries2. It involves stapling of the stomach to create a small gastric pouch, and connecting this portion to jejunum to form a gastrojejunal anastomosis (GJA). Although diet and lifestyle factors play a role in weight regain after RYGB, anatomic changes also play a role6–10. Dilated GJA aperture is an independent predictor of weight regain with a linear association11,12.
With the increasing number of patients who have undergone RYGB, weight regain, and return of comorbid conditions is of major concern. Endoscopic sutured transoral outlet reduction (TORe) is safe and effective in management of dilated GJA. This procedure has predominantly been performed by either (1) placing interrupted sutures at the GJA or (2) the creation of a pursestring (Figure 1)13–16. The latter technique is more technically challenging, however, provides more precise control of the final aperture, and has proven successful as a closure technique in the surgical literature17.
Figure 1.
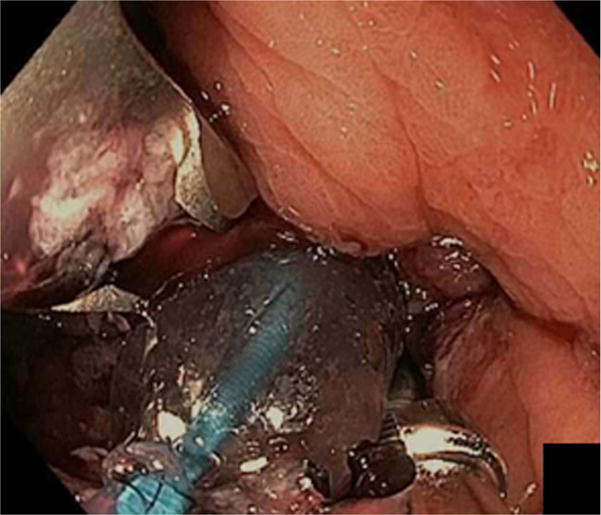
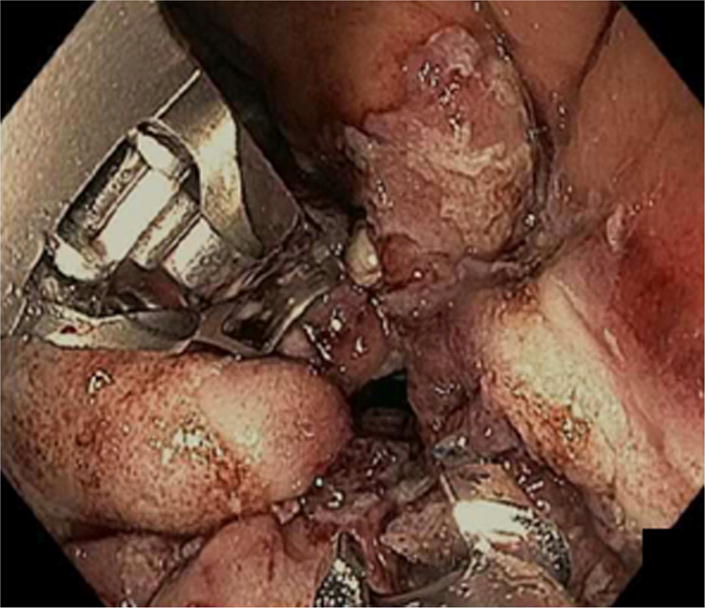
Endoscopic sutured transoral outlet reduction (TORe) performed by either placing interrupted sutures at the GJA (A) or the creation of a pursestring (B).
The aim of the current study was to compare the relative effectiveness of TORe using an interrupted suture technique versus a pursestring approach for therapy of weight regain in post-RYGB patients.
METHODS
Patients
All consecutive patients undergoing TORe using an interrupted stitch approach or pursestring pattern for weight regain after RYGB between 2010 and 20167 were prospectively enrolled in a bariatric database. To be eligible for TORe, patients were required to have a minimum of six months of pre-procedure bariatric visits with unsuccessful weight loss despite a structured diet and lifestyle modification plan, in addition to a dilated GJA over 15 mm in diameter. Patients were included if they reached the 12-month follow-up time point. Patients who underwent either endoscopic submucosal dissection (ESD) before suture placement or a double pursestring suturing pattern at the time of TORe were not eligible for inclusion. The study was approved by the Partners Healthcare Institutional Review Board (IRB) before inception (2003P001597).
Endoscopic suturing preparation
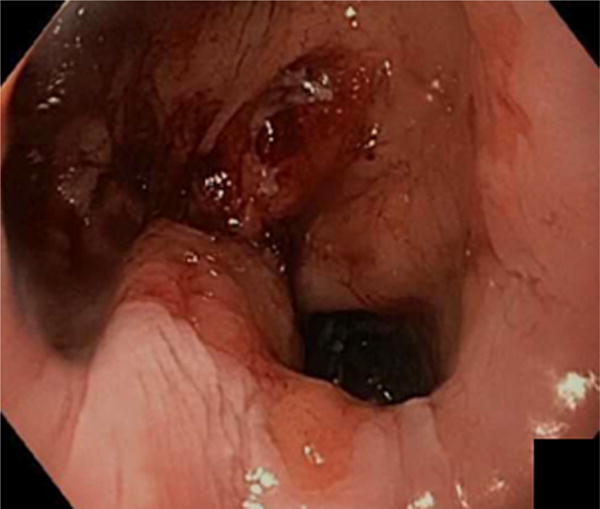
The initial portion of the procedure before endoscopic suturing was identical in both arms. Patients underwent bowel preparation the night before the procedure (Golytely or Miralax) to prevent intraprocedure bowel movements, post-procedure constipation and straining, and to minimize abdominal discomfort. General anesthesia with endotracheal intubation was used and all procedures were performed with C02 insufflation. Upper endoscopy was performed to estimate GJA aperture and pouch length, and confirm no new contraindications to undergoing endoscopic suturing including ulcerations. An overtube (Guardus, U.S. Endoscopy, Mentor, Ohio) was then inserted, and 60 milliliters of simethicone was instilled down the distal roux limb to minimize abdominal bloating and distention after the procedure18. A 5- to 10–mm area around the margin of the GJA was ablated before suturing by using end-firing forced argon plasma coagulation at 30 watts and flow rate of 0.8L/min (Figure 2).
Figure 2.
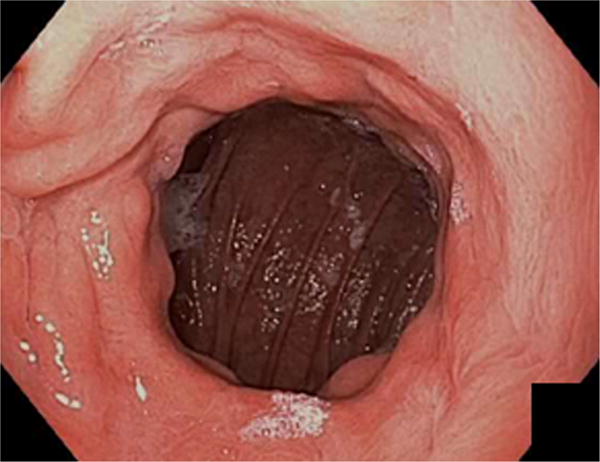
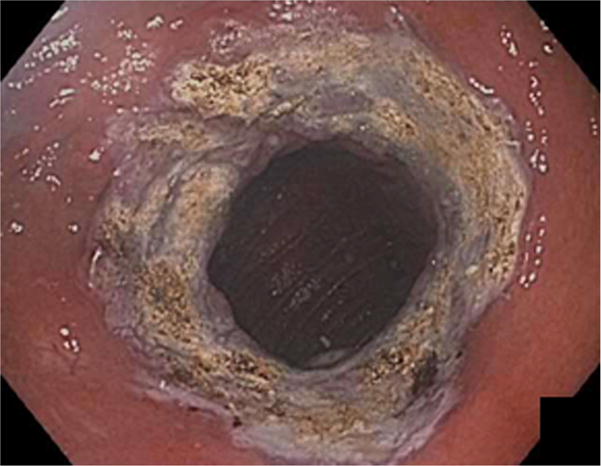
Dilated gastrojejunal anastomosis (A) after ablation around the margin of the GJA before suturing by using end-firing forced argon plasma coagulation.
Endoscopic suturing device
In both groups, TORe was performed by using the Apollo OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, Tex) (Figure 3) with polypropylene non-absorbable surgical suture (2-0 DemeLENE). The device attaches to a double-channel endoscope (GIF-2T160; Olympus America, Center Valley, Pa). It is composed of a curved suture arm attached to the endoscope tip, and an anchor exchange which is deployed through one channel. With activation of the handle, the suture arm propels the needle through the gastric tissue and passes the needle to the anchor exchange. Opening of the handle then releases the tissue. The anchor then can be passed back to the suture arm in anticipation of additional stitch placement.
Figure 3.

OverStitch endoscopic suturing device (A). The handle is attached to the double-channel upper endoscope, and the anchor exchange catheter is advanced through the larger accessory channel (B). The distal attachment is secured to the tip of the endoscope, with needle and suture attached to the needle driver arm. A helical tissue grasper is positioned through the smaller accessory channel.
(Courtesy of Apollo Endosurgery, Incorporated, Austin, Texas; with permission)
Interrupted stitch pattern
In the interrupted group, individual sutures were placed sequentially from the lower left to the upper right of the GJA until the size of the aperture was reduced to 12 mm or less (Figure 4A). If the gastric pouch was dilated, interrupted stitches were placed in the distal pouch to reduce pouch volume and reinforce the anastomosis.
Figure 4.
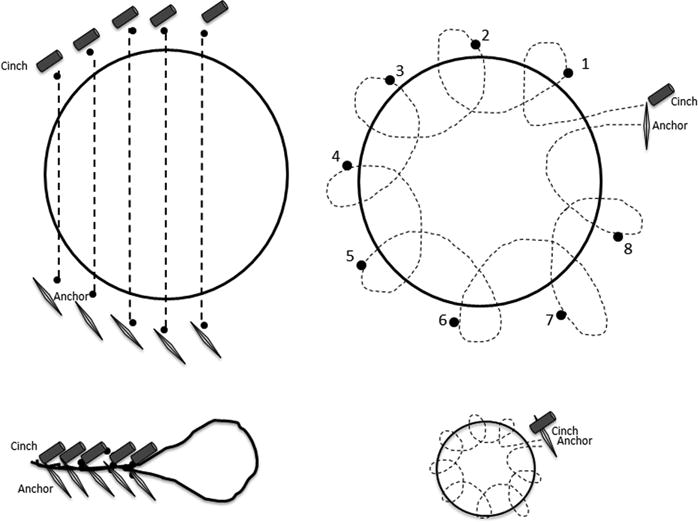
Schematic demonstrating outlet reduction using an interrupted suture pattern (A) or pursestring pattern (B) with final appearance after cinching in the interrupted pattern (C) or pursestring pattern (D).
Pursestring group
In the pursestring group, a single suture was placed around the margin of the GJA in a continuous ring. The running pursestring stitch was started after a full rotation to the 3 o’clock position and was continued in a counterclockwise fashion typically requiring 8 to 12 stitches (or bites) with a single suture. A hydrostatic dilation balloon (CRE balloon dilator, Boston Scientific, Marlborough, Mass) was passed through the second channel of the endoscope. It was inflated to a diameter of 8 to 12 mm inside the anastomosis (Figure 4B). The single suture was then tightened and cinched over the balloon, as previously described (Figure 4C)16. This technique provides precise and consistent control over the final anastomotic aperture, avoiding excessive or insufficient restriction. It also reinforces the entire margin of the anastomosis. Additional stitches were placed in the distal gastric pouch just proximal to the anastomosis to reinforce the pursestring suture.
After endoscopic suturing
After removal of suturing equipment, a standard upper endoscope was then advanced through the overtube. Close examination of the gastrojejunal anastomosis was performed to confirm suture integrity. Inspection of the esophagus after overtube removal was performed to ensure no bleeding or laceration.
Post-procedure
Patients had planned admission to the hospital for one day after the procedure. They were given nothing by mouth that evening. Nausea and abdominal pain were treated at the discretion of the primary inpatient team by intravenous medication. On the first day after the procedure, patients proceeded to a clear liquid diet for one day, followed by full liquids for 6 weeks. During this time, all medications were given in a crushed, chewable, or soluble form. For the 2 weeks after the liquid diet (ie, weeks 7 and 8 postprocedure), patients were maintained on a soft solid diet, and then transitioned to solid food. Follow-up clinical examinations were performed at 3 months and 12 months, with variable follow-up in between. These visits entailed weight recording and interview regarding postprocedure symptoms, dietary and exercise habits, and satiety.
Outcomes
The primary outcome was percent total weight loss (%TWL) for the interrupted and pursestring groups at 3 and 12 months. Secondary outcomes included mean percent excess weight loss (%EWL), percent regained weight lost (%RWL), and total weight loss (kilograms) for interrupted versus pursestring groups at 3 and 12 months.
Statistical analysis
Proportions were compared using fisher’s exact test. Continuous variables were assessed using the Student t-test or Wilcoxon for parametric or non-parametric data, respectively. Statistics were reported as mean ± SEM or median ± interquartile range (IQR). A p-value of 0.05 was significant. Multivariable regression analysis was performed to determine whether technique (ie, interrupted or pursestring approach) was an independent predictor of %TWL when controlling for known predictors of %TWL after TORe. Sensitivity analyses were also performed to determine whether final aperture in pursestring group affected %TWL.
RESULTS
A total of 241 patients underwent TORe, 210 of whom were female. Mean age was 50.3±10.7 years. Mean pre-RYGB body mass index (BMI) was 52.1±10.8 kg/m2. Postoperative nadir BMI was 31.3±7.5 kg/m2, and mean BMI at time of TORe was 40.1±9.2 kg/m2. TORe was performed 8.5 ±3.5 years after RYGB. Mean GJA aperture was 23.5±5.9 mm and mean pouch length was 4.9±1.8 cm. Baseline differences between groups are shown in Table 1. There was no statistical difference in pouch size between the pursestring and interrupted groups(4.6 ± 1.8 and 5.1 ± 1.8, p=0.37).
Table 1.
Baseline characteristics of patients included in study.
| Interrupted (n=54) | Pursestring (n=187) | P value | |
|---|---|---|---|
| Age | 50.9 ± 10.2 | 50.1 ± 10.7 | 0.67 |
| %EWL after RYGB | 68.4 ± 2.3 | 68.0 ± 1.4 | 0.88 |
| % Regained weight after RYGB | 42.4 ± 3.7 | 51.5 ± 3.8 | 0.11 |
| Pre TORe BMI | 42.2 ± 10.8 | 40.0 ± 8.5 | 0.22 |
| Pre TORe GJA (mm) | 23.8 ± 6.4 | 23.4 ± 5.5 | 0.59 |
| PreTORe pouch (mm) | 5.1 ± 1.8 | 4.6 ± 1.8 | 0.37 |
denotes p-value <0.05
There were 187 and 54 patients in the pursestring and interrupted groups, respectively. Of these, 131 (71%) and 44 (82%) were followed-up at 1 year. One patient in the pursestring group was excluded because she became pregnant shortly after the procedure.
Total number of stitches (or bites) to reduce the size of the GJA did not differ between the pursestring and interrupted groups (8.7 vs 9.7, p = 0.70). There was no significant difference between the number of sutures placed to reduce pouch volume in the pursestring and interrupted groups (1.9 vs 1.4, p = 0.27).
Weight loss outcomes including %TWL, %EWL, % RWL, and total weight loss, and at three and 12 months are shown in Table 2. There was no statistical difference between the pursestring and interrupted group at 3 months in %TWL (8.6 vs 8.0, p=0.41), %EWL (20.5 vs 16.7, p=0.39), % RWL (44.7 vs 33.3, p=0.56), and total weight loss (9.5 vs 11.3, p=0.32). At 12 months, the pursestring group achieved statistically significant improvement in %TWL (8.6 vs 6.4, p=0.02), %EWL (19.8 vs 11.7, p=<0.001), % RWL (40.2 vs 27.8, p=0.02), and total weight loss (9.5 vs 7.8, p=0.04).
Table 2.
Procedure characteristics and outcome differences between patients undergoing interrupted versus pursestring suturing patterns.
| Interrupted (n=48 at 3 mo, 44 at 12 mo) |
Pursestring (n=164 at 3 mo, 131 at 12 mo) |
P value | |
|---|---|---|---|
| Stitches, GJA | 9.7 ± 5.6 | 8.7 ± 2.8 | 0.70 |
| Percent total weight loss (%TWL) | |||
| 3 months | 8.0 [6.5, 9.4] | 8.6 [6.8, 9.4] | 0. 41 |
| 12 months | 6.4 [4.7, 8.1] | 8.6 [7.3, 9.4] | 0.02* |
| Percent Excess weight loss (%EWL) | |||
| 3 months | 16.7 [11.0, 21.3] | 20.5 [18.0, 23.0] | 0.39 |
| 12 months | 11.7 [5.8, 20.0] | 19.8 [16.4, 23.0] | <0.0 01* |
| Total weight loss (kg) | |||
| 3 months | 11.3 [6.7, 17.7] | 9.5 [8.6, 10.5] | 0.32 |
| 12 months | 7.8 [5.5, 9.3] | 9.5 [7.7, 11.2] | 0.04* |
| Percent regained weight lost (%RWL) | |||
| 3 months | 33.3 [23.5, 56.0] | 44.7 [24.5, 65.1] | 0.56 |
| 12 months | 27.8 [11.4, 60.0] | 40.2 [31.9, 48.5] | 0.02* |
denotes p-value <0.05
There was no difference in %TWL when final aperture size was assessed as a continuous variable (p=0.8371). Furthermore, no differences in %TWL were found on sensitivity analyses performed in the pursestring group on final aperture size.
Results from univariable and multivariable regression analyses are shown in Table 3. Multivariable regression showed technique (p=0.006) was an independent predictor of %TWL, when adjusting for age, sex, percent weight regain after initial RYGB, and pre-TORe GJA size. Additional multivariable models demonstrated that pouch size was not an independent predictor of %TWL.
Table 3.
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Risk Factors | Beta estimate (95% CI) |
P value | Adjusted Beta estimate (95% CI) | P value |
| Age | −0.02 ± 0.05 | NS | 0.02 ± 0.10 | NS |
| Male | 1.93 ± 0.61 | NS | 2.34 ± 1.59 | NS |
| % Regain after initial RYGB | 0.03 ± 0.01 | <0.01* | 0.03 ± 0.01 | <0.01* |
| Technique (interrupted = reference) | 3.20 ± 1.23 | 0.01* | 3.51 ± 1.26 | <0.01* |
| Pre-GJA size | −0.19 ± 0.21 | NS | 0.05 ± 0.09 | NS |
There were 2 major adverse events which included one marginal ulceration with a visible vessel that was successfully treated with hemoclip placement and without the need for transfusion, and 1 gastrojejunal stricture requiring 3 sessions of balloon dilation. Minor adverse events include 5 patients with marginal ulceration without stigmata of recent bleeding, and 5 patients with esophageal abrasions or tears that were prophylactically clipped.
DISCUSSION
Weight regain after RYGB is a serious and growing concern given the cumulative number of patients undergoing this procedure. Surgical revision is controversial, as it carries significant risk19. Moreover, the degree of weight regain does not always justify surgical intervention.
Technological advancement in the field of bariatric endoscopy has led to new innovative approaches for decreasing the size of the gastrojejunal anastomosis. Level 1 evidence now supports the short term efficacy of TORe for the management of weight regain after RYGB, and recent studies have also demonstrated this procedure to be safe and effective in the long-term13,20. Techniques to perform TORe and optimize weight loss are evolving.
A variety of approaches have been investigated for achieving outlet reduction13,16,20–22. Full-thickness suturing appears to be more effective than superficially-placed stitches, however, no studies have compared differences in stitch pattern performed at the time of outlet reduction. This is the largest, prospective study to date and demonstrates that the creation of a pursestring results in more weight loss at 1 year than the traditional interrupted suture pattern. Although the pursestring technique requires more stitches (or bites), rotation, and avoidance of stitch crossing, making it potentially more technically challenging, it provides reinforcement of the entire margin of the anastomosis, and also results in more precise control of the final aperture. Additionally, the reinforcing stitches just proximal to the GJA protect this area while it is healing.
In the present study, %TWL, %EWL, %RWL, and total weight loss (kilograms) were similar between groups at 3 months, however, at 1 year were all significantly better in patients undergoing the pursestring technique as compared to the interrupted approach, suggesting greater durability of this approach. Multivariable regression analysis demonstrated that pursestring technique was an independent predictor of %TWL. Furthermore, percent of weight regained after RYGB, a known predictor of response to TORe in previous studies, was similarly found to be a predictor in our model, further strengthening our results. Given the decreased invasiveness and morbidity associated with TORe, this procedure should be considered a part of the multidisciplinary strategy for management of this condition. More specifically, based on the results of this study, we would recommend the pursestring technique over the traditional interrupted suture pattern.
There are several limitations to this study. First, all of the procedures were performed at a single center. As a large referral center for post-bariatric surgery patients, a standard protocol is employed for endoscopic and post-endoscopic management. This did not change over the time period of the study. Therefore, the adjustment in suture pattern represents the only difference between these two groups. Procedures involving the traditional interrupted stitch approach were mostly performed between 2010 and 2012, whereas pursestring procedures were typically performed between 2013 and 2016, based on evidence that it may be a more effective procedure. We do not believe a learning curve was involved with the interrupted series because the investigators had extensive experience with endoscopic suturing since 2003 including use of the Eagle claw (predecessor to the overstitch device) and the first generation overstitch device. There may have been a learning curve involved with early pursestring suturing because this was a new stitch pattern at the time. However, this would likely bias against our conclusion. Finally, follow-up endoscopy was rarely performed due to lack of clinical indication and insurance approval, so durability of aperture size and suture retention was unable to be assessed.
In summary, creation of a pursestring during TORe results in more weight loss at twelve months than the traditional interrupted suture pattern. The pursestring suture pattern should be the preferred method of gastric bypass revision.
Abbreviations
- RYGB
Roux-en-Y gastric bypass
- GJA
Gastrojejunal anastomosis
- TORe
Endoscopic sutured transoral outlet reduction
- %TWL
Percent total weight loss
- %EWL
Percent excess weight loss
- %RWL
Percent regained weight lost
- BMI
Body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Schulman AR: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
N Kumar: study concept and design; acquisition of data
Thompson CC: study concept and design; critical revision of the manuscript for important intellectual content; study supervision
Financial Disclosures: A. Schulman - has no personal or financial conflicts of interest to disclose. CC Thompson – Apollo Endosurgery, Inc. (Consultant/Research Support); Olympus (Consultant/Research Support); Boston Scientific (Consultant); Covidien (Consultant, Royalty, Stock).
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA [Internet] 2004 Oct 13;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [cited 2016 Aug 17]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/15479938. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Oien DM. Metabolic/Bariatric Surgery Worldwide 2011. Obes Surg [Internet] 2013 Apr 22;23(4):427–36. doi: 10.1007/s11695-012-0864-0. [cited 2017 Jan 14]; Available from: http://link.springer.com/10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 3.Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS, et al. Association Between Bariatric Surgery and Long-term Survival. JAMA [Internet] 2015 Jan 6;313(1):62. doi: 10.1001/jama.2014.16968. [cited 2017 Jan 14] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25562267. [DOI] [PubMed] [Google Scholar]
- 4.Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg [Internet] 2008 Jan;247(1):21–7. doi: 10.1097/SLA.0b013e318142cb4b. [cited 2017 Jan 14] Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-200801000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Colquitt JL, Clegg AJ, Loveman E, Royle P, Sidhu MK. Surgery for morbid obesity. In: Colquitt JL, editor. Cochrane Database of Systematic Reviews [Internet] Chichester, UK: John Wiley & Sons, Ltd; 2005. p. CD003641. [cited 2017 Jan 14] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16235331. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti CF, de Oliveira BAP, de Pinhel MAS, Donati B, Marchini JS, Salgado Junior W, et al. Influence of Excess Weight Loss and Weight Regain on Biochemical Indicators During a 4-Year Follow-up After Roux-en-Y Gastric Bypass. Obes Surg [Internet] 2015 Feb 5;25(2):279–84. doi: 10.1007/s11695-014-1349-0. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24996801. [DOI] [PubMed] [Google Scholar]
- 7.Christou NV, Look D, MacLean LD. Weight Gain After Short- and Long-Limb Gastric Bypass in Patients Followed for Longer Than 10 Years. Ann Surg [Internet] 2006 Nov;244(5):734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term Weight Regain after Gastric Bypass: A 5-year Prospective Study. Obes Surg [Internet] 2008 Jun 8;18(6):648–51. doi: 10.1007/s11695-007-9265-1. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18392907. [DOI] [PubMed] [Google Scholar]
- 9.Barhouch AS, Padoin AV, Casagrande DS, Chatkin R, Süssenbach SP, Pufal MA, et al. Predictors of Excess Weight Loss in Obese Patients After Gastric Bypass: a 60-Month Follow-up. Obes Surg [Internet] 2016 Jun 4;26(6):1178–85. doi: 10.1007/s11695-015-1911-4. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26433591. [DOI] [PubMed] [Google Scholar]
- 10.Lim CSH, Liew V, Talbot ML, Jorgensen JO, Loi KW. Revisional Bariatric Surgery. Obes Surg [Internet] 2009 Jul 30;19(7):827–32. doi: 10.1007/s11695-008-9750-1. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18972173. [DOI] [PubMed] [Google Scholar]
- 11.Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal Stoma Diameter Predicts Weight Regain After Roux-en-Y Gastric Bypass. Clin Gastroenterol Hepatol [Internet] 2011 Mar;9(3):228–33. doi: 10.1016/j.cgh.2010.11.004. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21092760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Olalde ME, Rojano-Rodríguez ME, González-Angulo A, Morales-Chávez CE, Beristain-Hernández JL, Sierra-Murguía MA, et al. Correlation between the gastrojejunostomosis area, documented endoscopically, and the loss of weight in laparoscopic gastric bypass postoperative patients: results of 1 year after surgery. Surg Laparosc Endosc Percutan Tech [Internet] 2014 Aug;24(4):378–80. doi: 10.1097/SLE.0b013e3182901528. [cited 2017 May 19] Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00129689-201408000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Kumar N, Thompson CC. Transoral outlet reduction for weight regain after gastric bypass: long-term follow-up. Gastrointest Endosc [Internet] 2016 Apr;83(4):776–9. doi: 10.1016/j.gie.2015.08.039. [cited 2017 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26344204. [DOI] [PubMed] [Google Scholar]
- 14.Jirapinyo P, Slattery J, Ryan MB, Abu Dayyeh BK, Lautz DB, Thompson CC. Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy [Internet] 2013 Jul 25;45(7):532–6. doi: 10.1055/s-0032-1326638. [cited 2017 Jan 15] Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0032-1326638. [DOI] [PubMed] [Google Scholar]
- 15.Thompson CC, Ryou MK, Kumar N, Slattery J, Aihara H, Ryan MB. Single-session EUS-guided transgastric ERCP in the gastric bypass patient. Gastrointest Endosc [Internet] 2014 Sep;80(3):517. doi: 10.1016/j.gie.2014.06.011. [cited 2017 Jan 27] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25028276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar N, Thompson CC. Comparison of a superficial suturing device with a full-thickness suturing device for transoral outlet reduction (with videos) Gastrointest Endosc [Internet] 2014 Jun;79(6):984–9. doi: 10.1016/j.gie.2014.02.006. [cited 2017 May 19] Available from: http://linkinghub.elsevier.com/retrieve/pii/S001651071400128X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Qi X, Jiang B, Sha Y, Song D. Transumbilical endoscopic technique for complete closure of inguinal hernias in female pediatric patients. Exp Ther Med [Internet] 2017 Jan;13(1):41–4. doi: 10.3892/etm.2016.3919. [cited 2017 Sep 21] Available from: https://www.spandidos-publications.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson CC, Chand B, Chen YK, DeMarco DC, Miller L, Schweitzer M, et al. Endoscopic Suturing for Transoral Outlet Reduction Increases Weight Loss After Roux-en-Y Gastric Bypass Surgery. Gastroenterology [Internet] 2013 Jul;145(1):129–137.e3. doi: 10.1053/j.gastro.2013.04.002. [cited 2017 Jul 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23567348. [DOI] [PubMed] [Google Scholar]
- 19.Coakley BA, Deveney CW, Spight DH, Thompson SK, Le D, Jobe BA, et al. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis [Internet] 2008 Sep;4(5):581–6. doi: 10.1016/j.soard.2007.10.004. [cited 2017 May 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18065290. [DOI] [PubMed] [Google Scholar]
- 20.Thompson CC, Chand B, Chen YK, Demarco DC, Miller L, Schweitzer M, et al. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology [Internet] 2013 Jul;145(1):129–137.e3. doi: 10.1053/j.gastro.2013.04.002. [cited 2017 May 19] Available from: http://linkinghub.elsevier.com/retrieve/pii/S0016508513004939. [DOI] [PubMed] [Google Scholar]
- 21.Thompson CC, Slattery J, Bundga ME, Lautz DB. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc [Internet] 2006 Nov 5;20(11):1744–8. doi: 10.1007/s00464-006-0045-0. [cited 2017 Jan 15] Available from: http://link.springer.com/10.1007/s00464-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 22.Changela K, Ofori E, Duddempudi S, Anand S, Singhal S. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after bariatric surgery: Techniques and efficacy. World J Gastrointest Endosc [Internet] 2016 Feb 25;8(4):239. doi: 10.4253/wjge.v8.i4.239. [cited 2017 May 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26962406. [DOI] [PMC free article] [PubMed] [Google Scholar]


