Abstract
We investigated the ability of preferred classical music to activate the nucleus accumbens in patients with Major depressive disorder (MDD). Twelve males with MDD and 10 never mentally ill male healthy controls (HC) completed measures of anhedonia and depression severity, and listened to 90-second segments of preferred classical music during fMRI. Compared to HCs, individuals with MDD showed less activation of the left nucleus accumbens (NAcc). Individuals with MDD showed attenuation of the left NAcc response in later compared to earlier parts of the experiment, supporting theories that MDD involves an inability to sustain reward network activation. Counter intuitively, we found that NAcc activity during early music listening was associated with greater depression severity. In whole-brain analyses, anhedonia scores predicted activity in regions within the default mode network, supporting previous findings. Our results support theories that MDD involves an inability to sustain reward network activation. It also highlights that pleasant classical music can engage critical neural reward circuitry in MDD.
Keywords: ventral striatum, MDD, classical music, anhedonia, major depression, fMRI, mood induction
1. Introduction
Anhedonia, the inability to experience pleasure during previously enjoyed activities, is a core symptom of Major depressive disorder (MDD), along with negative mood (American Psychiatric Association, 2013; Austin, Mitchell, and Goodwin, 2001; Langenecker et al., 2005; Rock, Roiser, Riedel, et al., 2014; Treadway and Zald, 2011). Individuals with MDD have been found to have impairments in reward and motivation circuitry (Nestler et al., 2002), in particular the ventral striatum (Marchand and Yurgelun-Todd, 2010). The ventral striatum (VS) is involved in the hedonic response, and Positron Emission Tomography (PET) has revealed that dopamine release in the VS is associated with the subjective experience of pleasure (Drevets et al., 2001). The volume of the nucleus accumbens (NAcc), a sub-region of the VS, has been correlated with anhedonia and reduced response to reward in non-clinical samples (Wacker, Dillon, and Pizzagalli, 2009). Trait anhedonia has also been reported to be negatively correlated with pleasantness ratings of music stimuli and of activation of the NAcc, basal forebrain and hypothalamus (Keller et al., 2013). Indeed, there is evidence of VS hypoactivation in response to positive stimuli in MDD (Epstein et al., 2006), and VS hypoactivation in anticipation of monetary loss in unmedicated individuals with MDD has been reported to normalize following treatment antidepressant treatment (Stoy et al., 2012). Despite an accumulation of evidence for the importance of VS dysfunction in anhedonia, results of a randomized controlled trial of deep brain stimulation of the VS in patients with treatment-resistant depression (Dougherty et al., 2015) have been less than encouraging (but see Schlaepfer, 2015). Therefore further investigation of the neural mechanisms underpinning anhedonia in MDD is warranted.
A reduced capacity to experience pleasure may be an oversimplification of anhedonia in MDD. Evidence from fMRI studies suggests that individuals with MDD fail to sustain VS activity during positive affect. For example, Moses-Kolko and colleagues (2011) found that the VS habituated rapidly following receipt of reward in mothers with postpartum depression compared to healthy mothers. Another study reported that the ability of individuals with MDD to cognitively upregulate their positive affect in response to emotional slides was equal to healthy controls (HC) in the first half of the experiment, yet declined as the experiment progressed (Heller et al., 2009). Furthermore, this inability to sustain NAcc activity was associated with reduced self-reported positive affect in the MDD group. In a follow-up study Heller and colleagues (2013) subsequently found that self-reported anhedonic symptoms decreased following two months of antidepressant treatment. Furthermore, this decrease was significantly correlated with increases in sustained NAcc activity after the two-month period in the MDD group.
Functional neuroimaging studies have found that pleasant music activates the mesolimbic reward pathway, including the VS and NAcc, orbitofrontal cortex (OFC), anterior insula, subgenual anterior cingulate cortex, midbrain, amygdala and ventromedial prefrontal cortex (Blood and Zatorre, 2001; Blood, Zatorre, Bermudez, et al. 1999; Brown, Martinez, and Parsons, 2004; Koelsch, Fritz, Von Cramon, et al., 2006; Menon and Levitin, 2005; Suzuki et al., 2008), in HCs, in addition to eliciting strong, pleasurable emotional states (Blood and Zatorre, 2001; Krumhansl, 1997). Degree of activity in mesolimbic reward regions, particularly the NAcc, during music heard for the first time, has been found to predict the amount of money that participants were willing to spend to purchase the music in an auction (Salimpoor et al., 2013). DA is released in the dorsal and ventral striatum in response to pleasant music, and degree of release is related to amount of pleasure experienced (Salimpoor, Benovoy, Larcher, et al., 2011).
The ability for music to modulate activity in the reward network has implications for the potential use of music therapy as a complementary treatment for MDD, or as an affective probe (Koelsch, 2014). Indeed, music therapy already shows promise as a treatment for MDD, (Castillo-Perez, Gomez-Perez, Velasco, et al., 2010; Maratos, Gold, Wang, et al., 2008). At this time, the neural mechanisms via which music exerts its beneficial effects remain uncertain, as few studies have examined neural activity in response to music in individuals with MDD.
Multiple small studies of music have been used as probes of emotion, reward, and of disrupted responsiveness in neural responses for those with MDD. Patients with MDD showed reduced activity in the NAcc/VS and medial OFC when listening to their favourite music compared to neutral music, despite similar ratings of enjoyment (Osuch et al., 2009). Lepping et al. (2016) recently reported no differences between MDD and HC groups in striatum activity in an fMRI study of emotion. However these researchers defined the striatum using a large ROI that incorporated the amygdala, which has been reported to be hyperactive in response to emotional stimuli in MDD (e.g. Fu et al., 2008; Sheline et al., 2001), thus potentially counterbalancing any NAcc hypoactivity. Lepping et al. (2016) did report a significant group difference in valence in the rostral anterior cingulate cortex, whereby the MDD group demonstrated greater activity following negative stimuli and the HC group greater activity following positive stimuli; however this contrast included both musical and non-musical stimuli. Finally, treatment-naïve males with first-episode MDD showed decreased neural activity for both pleasant instrumental music and music that induced feelings of inquietude and unrest (Flores-Gutiérrez, Cervantes, et al., 2013) when compared to HCs. HCs showed activity in the bilateral caudate body and anterior insula that was not present in the MDD group, as well as activity in the left posterior parahippocampal gyrus during the pleasant music. It is possible that a wide network of regions implicated in reward responsivity is disrupted in those with MDD.
Based on the emerging literature reviewed above, we hypothesized that compared to healthy controls (HCs), patients with MDD would show reduced activity in the NAcc when listening to preferred classical music compared to noise. In addition, we also hypothesized that NAcc activity in individuals with MDD would be greater in the first versus second half of the experiment due to difficulty sustaining reward network activation (Heller et al., 2013; Heller et al., 2009). Third, we hypothesized that in addition to depression severity, greater anhedonia scores would predict reduced activity in the NAcc. We based our final exploratory hypothesis on findings that individuals with MDD show greater activity than HCs during emotional stimuli in the default mode network (DMN; Grimm et al., 2009; Sheline et al., 2009), which consists of dorsal and ventral medial PFC, as well as medial and lateral parietal and temporal cortices (Fox et al., 2005; Raichle et al., 2001). We hypothesized that anhedonia and depression severity would predict increased activity in the DMN in whole-brain analyses.
2. Methods
2.1. Participants
Participants were 22 males aged 18-45, including 12 with active MDD, diagnosed using the Structured Clinical Interview for DSM Disorders (SCID; American Psychiatric Association, 2013), and 10 never mentally ill healthy controls (HC). Groups were matched for age, t(16.93)= −0.91, p= .37. We chose to examine males only in this initial pilot study in consideration of previously reported sex differences in response to music (Koelsch, Maess, Grossmann, et al., 2003) as well as potential emotional response shifts based upon menstrual phase (Derntl, Kryspin-Exner, Fernbach, et al., 2008; Protopopescu et al., 2005), given findings of the effect of menstrual phase on cognition and neural activity in response to music (Sanders and Wenmoth, 1998).
Participants were included based upon a notation of enjoying classical music on a Musical Sensitivity Questionnaire (MSQ). The MSQ measured different genres (rock, pop, jazz, classical) of music liked (yes/no responses). Exclusion criteria were a history of alcoholism, drug abuse, head injury, neurological disorder or serious medical illness; professional musician; self-reported hearing-impaired or tone-deaf, and MRI contraindications. We also excluded those who declined any prior exposure to (or pre-MDD enjoyment in listening to) classical music (western). Participants taking antidepressant medication were not excluded, however were required to have not had a dose adjustment of any medication for a minimum of six weeks prior to testing. Two participants in the MDD group were currently taking antidepressants (both Zoloft, one for < 1 year and one for > four years), and one was receiving counseling therapy. Including medicated individuals was appropriate, because our goal was to use music as a reward circuitry probe in actively depressed subjects. Participants taking anti-psychotics, antiepileptic drugs, benzodiazepines, or medications that act directly on dopamine (e.g. Wellbutrin) were excluded. This work was carried out in accordance with the Declaration of Helsinki, and informed consent was obtained from all participants.
2.2 Measures and Stimuli
2.2.1. The Snaith-Hamilton Pleasure Scale
The Snaith-Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995) is a self-report instrument that was used as a measure of anhedonia, and is a reliable, valid and unidimensional measure of hedonic capacity in adult outpatients with MDD (Nakonezny, Carmody, Morris, et al., 2010).
2.2.2. The Hamilton Rating Scale for Depression
The Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1960) is a clinician-rated instrument that was used to assess depression severity.
2.2.3. Classical music stimuli
Classical music stimuli were used to elicit measurable changes in brain activation in response to pleasurable stimuli. Music excerpts were chosen based on pleasure ratings by a focus group of 15 individuals in a science research group. Eight 90-second nonvocal excerpts were selected: Mozart (Piano Concerto 23; Eine kleine nachtmusik; Le nozze di Figaro), Bach (two excerpts of Brandenburg concerto no. 3), Beethoven (Violin romance no. 2), Bizet (Carmen), and Schumann (Symphony no. 1) from an initial larger set of over 20 selections. Classical music stimuli of 90 seconds duration has been previously reported to elicit regional cerebral blood flow (rCBF) in reward and emotion regions (Blood and Zatorre, 2001). Ninety-second blocks of noise (white, gray, pink, and brown) were used as a control condition matched for decibel range and auditory processing. Music and noise clips were played through MRI-compatible pneumatic earphones - scanner noise was controlled for by placing noise-cancelling headphones outside of these earphones. Volume was adjusted prior to the scanning beginning and again as needed to maintain listener comfort.
2.3. Procedure
On day 1, participants completed the SCID and Musical Sensitivity Questionnaire. They also listened to and rated all 8 classical music excerpts for pleasure and arousal experienced on separate 5-point Likert scales. Their top four clips, based upon pleasure ratings were used in the fMRI experiment. Mann Whitney U tests found no significant differences between groups in the pleasure ratings of the four clips heard within the scanner (p> .05).
On a second day, participants underwent fMRI while listening to their four top-rated 90 second excerpts of music and the four noise clips. Each selection was heard twice during the fMRI experiment. Fisher’s exact tests found no significant differences between groups in the proportion of individuals that listened to each clip in the scanner (p> .05). Music clips were played in a randomly assigned order that alternated between music and noise, and each music clip was heard in each half of the scan. Between music and noise clips in the scanner, participants completed cognitive and emotional tasks, the results of which will be reported in a separate publication.
2.4 fMRI measurement and data analysis
A 3T GE Signa scanner acquired images using a forward reverse spiral functional sequence. Each volume comprised 36 slices. The TR was 1.75 for the first half (four runs) of the scan and 2 for the second half (four runs) of the scan. T1spgr and T1overlays were collected for coregistration purposes. fMRI data was analysed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). SPM8, AFNI (http://afni.nimh.nih.gov/afni/) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) were used to preprocess fMRI data. Data were despiked using AFNI, slice-time corrected in SPM, then realigned to the 10th volume in FSL using MCFLIRT (Jenkinson, Bannister, Brady, et al., 2002). Brain extraction of anatomical images was performed with FSL’s Brain Extraction Tool (Smith, 2002), then co-registered to functional images and spatially normalized to Montreal Neurological Institute (MNI) space in SPM, with a final reconstructed spatial resolution of 2 × 2 × 2. Smoothing was completed in SPM with a full-width at half-maximum filter of 5mm. The subtraction method was used to create contrast images in SPM8. The blood oxygen level dependent (BOLD) signal for the noise blocks was subtracted from the noise blocks (Music minus noise contrast, M-N) to examine music processing controlling for auditory processing. Two spherical ROIs (radius 5mm) were placed in the nucleus accumbens (NAcc) in each hemisphere (+/−9, 9, −8) to extract BOLD signal using Marsbar. Exploratory whole-brain analyses were also conducted. Whole-brain correction (p< .01) was estimated with Monte Carlo simulation using Alpha-Sim (peak threshold p< .005, extent threshold = 55). Linear regressions were performed on data (both whole brain and ROI) from the MDD group only with SHAPS and HAM-D scores as predictors. The HC group was not included in these regressions as every HC had a score of 0 on the SHAPS.
3. Results
3.1. Self-report ratings
On the HAM-D, the MDD group reported moderate depressive symptoms (M= 15.08, SD= 4.14, range=10 to 24) that were significantly higher than the HC group (M= 0.40, SD= 0.70), t(11.75) = −12.07, p< .001. On the SHAPS, the MDD group (M= 6.67, SD= 2.39, range = 4 to 11) scored significantly higher than the HC group (M= 0.00, SD= 0.00), t(11) = −9.68, p< .001.
3.2. fMRI
3.2.1. Group differences in nucleus accumbens activity
Data extracted from the NAcc was analysed using a 2 (hemisphere) × 2 (group) repeated measures ANOVA. There was a significant hemisphere by group interaction, F(1, 20)= 5.17, p= .034, η2p= .21. This interaction is shown in Figure 1, with group differences in left NAcc.
Figure 1.
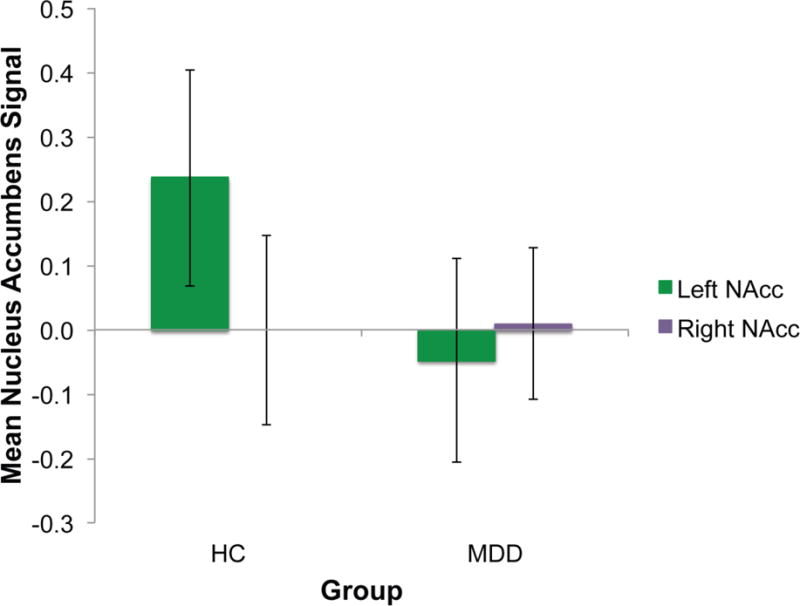
Extracted values from the left (green) and right (purple) nucleus accumbens averaged over the entire experiment.
3.2.2. Nucleus accumbens signal change over time
A priori analyses examined the effect of time on NAcc signal to test the hypothesis that NAcc signal would decrease over time in MDD. A delta (difference score) was calculated by subtracting the average NAcc from the second half of the experiment from the average NAcc from the first half of the experiment, for each hemisphere (Figure 2). One sample t tests found that in the MDD group, the left NAcc signal change (M= −0.52, SD= 0.78) was significantly different from zero, t(11)= −2.30, p= .04, however the right NAcc signal change (M= −0.33, SD= 0.70) was not, t(22)= −1.61, p= .14. A posthoc paired sample t test found that the difference in NAcc signal change between hemispheres was significant, t(11)= −2.32, p=.04. For the HC group, no changes were significant over time, nor were there hemisphere differences.
Figure 2.
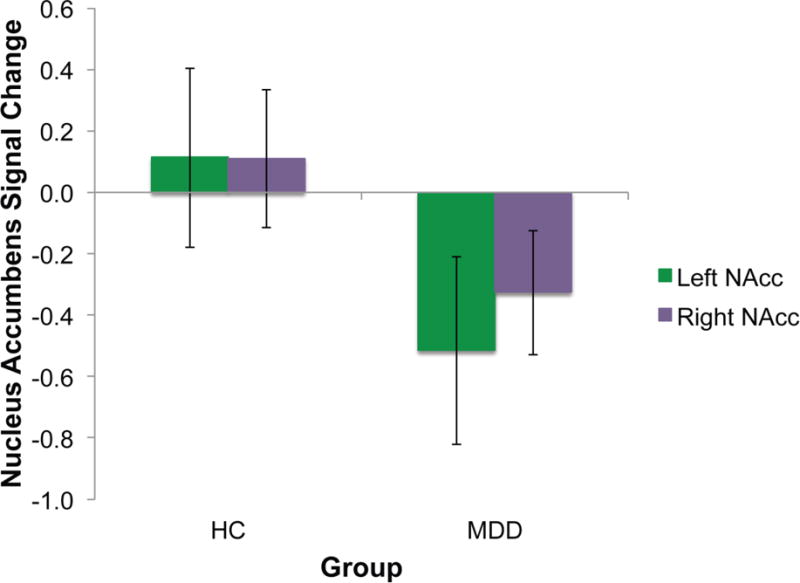
Signal change in the nucleus accumbens over two halves of the experiment.
3.2.3. NAcc signal relationship to depression severity and anhedonia
A stepwise multiple regression analysis of left NAcc activity during music minus noise (M-N) in the first half of the experiment was calculated for the MDD group only, with HAM-D score entered in the first step and SHAPS entered in the second step to determine whether anhedonia added any significant variance to left NAcc activity beyond that already contributed by depression severity.
The model was significant at the first step, R2= .39, F(1, 10)= 6.44, p= .03. Adding SHAPS to the model did not significantly increase the F value, F(1, 9)= 1.71, p= .22, however the overall model remained a significant predictor of left NAcc activity, F(2, 9)= 4.30, p= .049.
Surprisingly the relationship between HAM-D score and left NAcc activity was not in the expected direction. Rather, for MDD, HAM-D score was positively correlated with left NAcc activity during the first half of the experiment (r= .63, p< .029), although not the second. Figure 3 demonstrates these relationships, and includes the HC group for comparison only.
Figure 3.
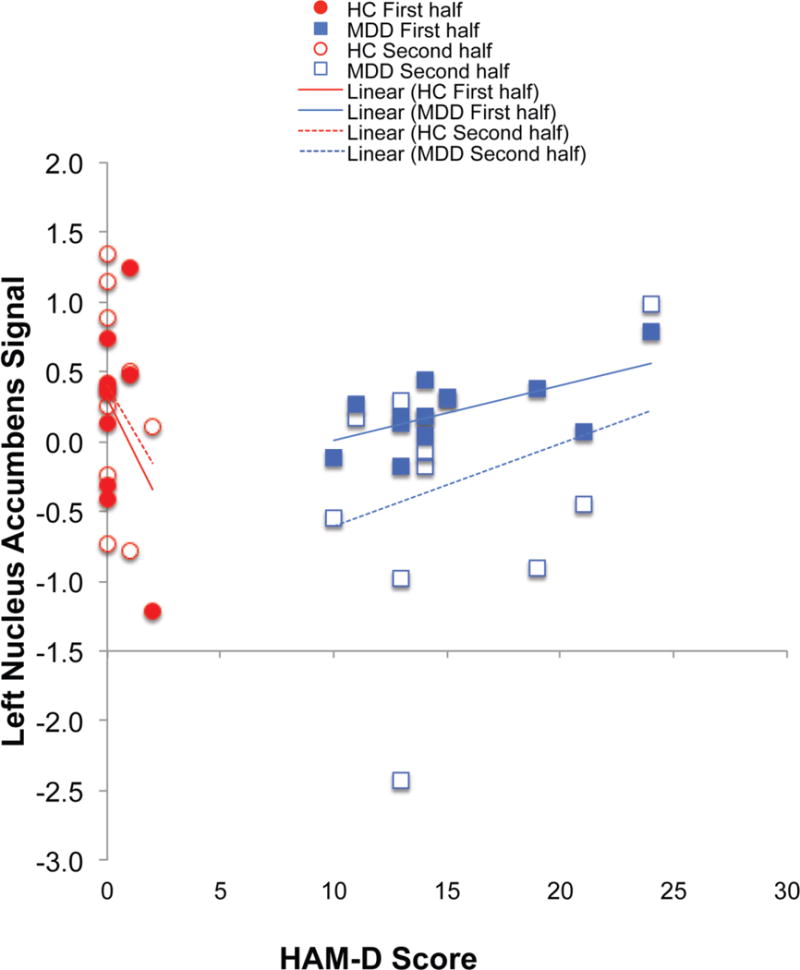
Scatterplot of scores on the HAM-D and signal in the left NAcc ROI during the first (solid) and second (open) halves of the experiment.
3.2.4. Whole-brain analyses
Exploratory whole brain analyses were also conducted for M-N. Figure 4 reveals that both groups demonstrated activity in the bilateral auditory cortex. The MDD group additionally showed activity in the medial and lateral frontal cortex in regions involved in attention and cognitive control, however there were there were no significant differences in activity between groups for M-N.
Figure 4.
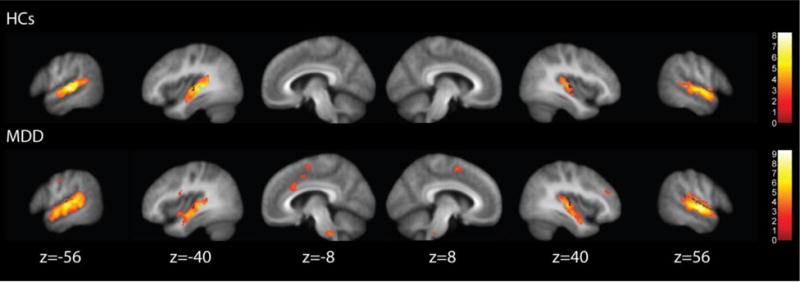
Results of the music minus noise contrast.
3.2.5. Whole brain multiple regression analyses
A multiple regression analysis was calculated for the MDD group only using SHAPS score as a predictor of whole brain activity for the M-N contrast during both first and second halves of the experiment. SHAPS scores were positively related to activity in default mode network (DMN) regions including the posterior cingulate cortex and precuneus, as well as some cognitive control network (CCN) regions, including the inferior frontal gyrus (see Table 1 and Figure 5). Another whole-brain multiple regression analysis found that HAM-D scores were also positively related to activity in some DMN regions (e.g. precuneus) and also more CCN regions such as the dorsolateral prefrontal cortex and inferior frontal gyrus.
Table 1.
Regressions of SHAPS and HAM-D onto whole-brain activity in MDD only.
| Regressor | Lobe/gyrus | BA | MNI | Peak | Cluster | ||
|---|---|---|---|---|---|---|---|
| x | y | z | Z | k | |||
| SHAPS | Frontal | ||||||
| precentral | 4 | 6 | −24 | 74 | 4.33 | 346 | |
| 4 | −2 | −20 | 66 | 3.49 | 308 | ||
| inferior (pars triangularis) | 45 | 54 | 20 | 2 | 3.74 | 77 | |
| inferior (pars opercularis) | 44 | 54 | 20 | 26 | 3.48 | 77 | |
| superior | 9 | 4 | 60 | 16 | 3.52 | 82 | |
| 9 | −18 | 46 | 34 | 3.37 | 64 | ||
| Parietal | |||||||
| angular | 39 | 60 | −52 | 26 | 3.39 | 74 | |
| cingulate (splenium) | 26 | 8 | −44 | 8 | 3.29 | 122 | |
| precuneus | 7 | −6 | −64 | 42 | 3.23 | 133 | |
| Temporal | |||||||
| middle | 21 | −64 | −52 | −2 | 3.31 | 118 | |
| middle | 21 | 62 | −6 | −18 | 3.09 | 64 | |
| inferior | 20 | 48 | −52 | −18 | 3.73 | 228 | |
| Cerebellar tonsil | – | −10 | −62 | −34 | 4.00 | 141 | |
|
| |||||||
| HAM-D | Frontal | ||||||
| superior | 6 | −20 | 20 | 48 | 3.91 | 338 | |
| middle | 10 | −32 | 48 | 6 | 3.94 | 155 | |
| inferior | 11 | 28 | 36 | −14 | 3.44 | 89 | |
| Parietal | |||||||
| precuneus | 7 | −4 | −72 | 42 | 3.32 | 57 | |
| precuneus | 7 | 16 | −66 | 30 | 3.04 | 61 | |
| Temporal | |||||||
| superior | 42 | 64 | −32 | 6 | 4.05 | 310 | |
Figure 5.
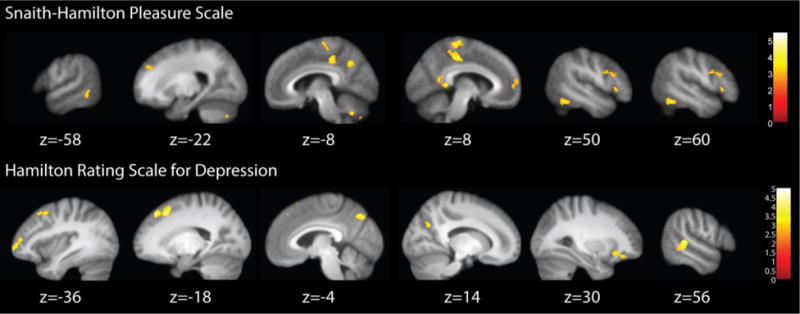
Results of the regressions of Snaith-Hamilton Pleasure Scale (top) and Hamilton Rating Scales for Depression (bottom) onto whole brain activity during Music minus Noise for the MDD group only.
4. Discussion
The present study aimed to measure neural activity in response to preferred classical music in individuals with MDD compared to HCs, and to examine it’s relationship to anhedonia and depression severity. We hypothesised that patients with MDD would show reduced NAcc activity compared to HCs when listening to preferred classical music compared to noise. Second, we hypothesized that individuals with MDD would show reduced NAcc activity in the second compared to the first half of the experiment. Third, we hypothesized that anhedonia scores would predict reduced NAcc activity over and above that of depression severity. Finally, we hypothesized that anhedonia and depression severity would predict activity in the default mode network in individuals with MDD. These results are discussed in turn.
For NAcc activity over the entire experiment, we observed a significant interaction between group and hemisphere, offering partial support for our hypothesis of reduced NAcc activation during preferred classical music in MDD. This finding supports that of Epstein et al. (2006) who reported that compared to HCs, patients with MDD had reduced ventral striatum activity in response to positive stimuli (words). However, these researchers found the VS hypoactivation to be bilateral, whereas our finding was restricted to the left hemisphere. The effect of the right NAcc was in similar direction to left NAcc, but the study was likely underpowered to achieve significance. In contrast, hypoactivation of the left VS has been previously reported in MDD during anticipation of positive and negative monetary incentives (Stoy et al., 2012). Left NAcc hypoactivity in MDD has also been reported during the consummatory phase of reward processing during such monetary incentive delay tasks (Pizzagalli et al., 2009). Left VS hypoactivation has also been reported in mothers with postpartum depression in response to receipt of monetary reward (Moses-Kolko et al., 2011). Thus, our pilot finding, in combination with the growing evidence implicating left VS hypoactivity in experimental manipulation of reward processing, offers converging support for the argument that deficient engagement of the left NAcc in particular is important for alterations in reward processing such as anhedonia, observed in MDD.
Our hypothesis that NAcc activity in MDD would attenuate over the course of the experiment was supported. The finding of reduced activation during the second presentation of the identical music selection was driven by a significant reduction in the left NAcc signal. This supports a prior study of mothers with postpartum depression that reported a greater attenuation of left ventral striatum response to reward over time compared to healthy mothers (2011). Sustained NAcc activity to pleasant stimuli may be a marker of increased positive affect (Heller et al., 2013), whereas lack of sustained NAcc activity during the experience of positive stimuli or emotions could be more typical of active MDD (Heller et al., 2013; Heller et al., 2009).
Anhedonia scores did not predict NAcc activation over and above that of depression severity in any part of the task. This result was contrary to our third hypothesis, in that we expected anhedonia to contribute additional predictive value to NAcc hypoactivity beyond that of depression severity. Our results of music as the stimulus do not support Keller et al. (2013) who found that anhedonia scores correlated with activity in mesolimbic reward structures for pleasant, non-musical stimuli. Our results also differ from another study reporting that VS activity was negatively correlated with anhedonia but not depression severity (2005). These researchers utilized different measures of anhedonia and depression severity to those used in the present study, thus it is possible that our contrasting findings may reflect a discriminant validity problem between the HAM-D and SHAPS, which are moderately correlated and measure distinct but overlapping constructs (Nakonezny et al., 2010). We found that greater depressive symptoms predicted greater, rather than less NAcc activation in the first half of the experiment. This is in contrast to previous research that suggests that deep-brain stimulation of the NAcc in treatment-resistant depression leads to a reduction of depression and anhedonic symptoms (Bewernick et al., 2010). The NAcc is an area crucial in the experience of positive emotions and pleasure (Berridge and Robinson, 1998; Koob, 1992; Pecina, 2008). We suggest that listening to music during the task temporarily modulated NAcc activation in higher-severity MDD participants, activity that may have been suppressed prior to music. This counter-intuitive finding requires further study.
In support of our final hypothesis, anhedonia scores predicted default mode network (DMN) activity across both halves of the experiment, including in parts of the medial prefrontal cortex, posterior cingulate, precuneus and lateral temporal lobes (Yeo et al., 2011). Our finding that medial PFC activity during music minus noise was predicted by SHAPS score supports that of Osuch and colleagues (2009) who reported that the medial PFC was correlated with SHAPS score during favourite minus neutral music listening in patients with MDD. Failure to down-regulate the DMN may lead to increased negative self-referential processing in MDD (Sheline et al., 2009). In support of this argument, Hamilton and colleagues (2011) reported that in MDD, higher levels of maladaptive, depressive rumination were associated with DMN dominance. Our results could be interpreted to support the assertion that hyperactivity of the DMN may contribute to increased rumination and greater anhedonia in MDD. We also found increased activity in the cognitive control region of the dorsal cingulate in MDD during music minus noise, and that anhedonia was predicted by increased activity in CCN regions, including the inferior frontal gyrus. Together with the reduced left hemisphere NAcc activity in MDD, our findings also offer support for theories that anhedonia may result from excessive top down inhibitory control of the PFC over NAcc (e.g. Nestler et al., 2002).
There are some limitations to the present study. First, the sample size was small (MDD = 12, HC = 10), which can increase the risk for type 2 error (as well as type 1 error), which may have precluded a reliable estimate of the relationship between anhedonia and NAcc activation; however it was comparable to other similar studies (Flores-Gutiérrez et al., 2013; Osuch et al., 2009). Second, noise may have not been the best control condition for music; others have used unpleasant music (Koelsch et al., 2006) and neutral music (Osuch et al., 2009), but we wished to avoid a positive-negative comparison. Our findings in the nucleus accumbens for music minus noise in MDD across halves of the experiment could be a result of relative upward or downward shifts in music or noise across the experiment. For example, decreased music minus noise in the second half of the experiment could be related to elevated activation in response to noise. It is unlikely, though, that repeated listening to noise would result in greater relative activation in MDD – the expected direction might be of relatively greater decreases if the noise were increasing in irritation or negative valence for MDD compared to HC.
Despite these potential limitations, this pilot study is important because only a handful of fMRI studies have examined anhedonia in MDD (e.g. Keedwell et al., 2005; Misaki, Suzuki, Savitz, et al., 2016), even though anhedonia is a key symptom for DSM diagnosis of MDD. Furthermore, to our knowledge, Young et al. (2016) are the only other study that specifically sought to investigate anhedonia in response to pleasant music in MDD during fMRI. These researchers examined seed-based functional connectivity of the posterior ventromedial prefrontal cortex during pleasant music listening. They found that greater anhedonia in MDD was associated with reduced ability to modulate functional connectivity between the posterior VMPFC and reward and emotion-related regions, including the NAcc. The music clips that Young et al. utilized were 3-4 times shorter than those presented in our study, and future research could examine changes in these connectivity patterns over time. On the basis of our results, we would hypothesize that with increased duration of preferred music exposure, this VMPFC-reward modulation would reduce even further.
In closing, some commentary on the potential implications of these results for music as a neural probe in MDD or complementary treatment for MDD are offered. Initial small studies suggest music therapy is effective. For example, one prior randomized controlled trial has examined the clinical use of music therapy as a treatment for inpatients with MDD (Castillo-Perez et al., 2010) when compared with CBT. Another study examined music therapy in older adults living in the community compared with no music therapy (Chan et al., 2012). Moreover, a systematic review of five randomized controlled trials of music therapy in MDD found that music therapy in combination with standard therapy improved mood compared to standard therapy alone (Maratos et al., 2008). A recent meta-analysis has found dose-response relationships for music therapy for serious mental illness and treatment-resistant depression, such that response improved with more sessions of music therapy (Gold, Solli, Kruger, et al., 2009).
In a related way, our study suggests that music is a useful probe to examine the functioning of the NA and related circuits in MDD. As with other probes, we showed that activation by music in MDD is decreased compared to HC over time. Additional studies are needed to evaluate the differing time course of responsiveness in MDD. Our experiment differs on a number of levels from music therapy (type, dose, duration, setting). Further research is needed to better understand how to maximize the strength and duration of activation to increase the effectiveness of music in the treatment of major depressive disorder. Future studies could look at the long-term effects of repeated exposure to pleasant music and if this results in more sustained NAcc signal change and elevation of depression symptoms.
Supplementary Material
Highlights.
12 males with major depression (MDD) completed measures of anhedonia and depression
Also underwent fMRI whilst listening to preferred classical music
MDD group showed less activation of nucleus accumbens (NAcc) than healthy controls
MDD group showed attenuation of NAcc response in later compared to earlier times
Supports theories that MDD involves inability to sustain reward network activation
Acknowledgments
Funding: This work was supported by NIH UL1TR000433 and UL1TR002240 MICHR pilot grant to Monica Starkman and departmental support from The University of Michigan Department of Psychiatry to Drs Starkman and Langenecker.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. American Psychiatric Association; Washington DC: 2013. [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. Brit J Psychiat. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockman H, Lenartz D, Sturm V, Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiat. 2010;67(2):110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. P Natl Acad Sci USA. 2001;98(20):11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2(4):382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport. 2004;15(13):2033–2037. doi: 10.1097/00001756-200409150-00008. [DOI] [PubMed] [Google Scholar]
- Castillo-Perez S, Gomez-Perez V, Velasco MC, Perez-Campos E, Mayoral MA. Effects of music therapy on depression compared with psychotherapy. Art Psychother. 2010;37(5):387–390. doi: 10.1016/j.aip.2010.07.001. [DOI] [Google Scholar]
- Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm Behav. 2008;53(1):90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, Eskander EN, Baltuch GH, Machado AD, Kondziolka D, Cusin C, Evans KC, Price LH, Jacobs K, Pandya M, Denko T, Tyrka AR, Brelje T, Deckersbach T, Kubu C, Malone DA. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiat. 2015;78(4):240–248. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiat. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang YH, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiat. 2006;163(10):1784–1790. doi: 10.1176/appi.ajp.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Flores-Gutiérrez EO, Cervantes JJ, Álvarez MT, Solórzano SA. Processing of music in the first episode of major depressive disorder without treatment. Salud Mental. 2013;36(6):449–457. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. P Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiat. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Gold C, Solli HP, Kruger V, Lie SA. Dose-response relationship in music therapy for people with serious mental disorders: Systematic review and meta-analysis. Clin Psychol Rev. 2009;29(3):193–207. doi: 10.1016/j.cpr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacol. 2009;34(4):932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiat. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol, Neurosur Ps. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light S, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Relationships between changes in sustained fronto-striatal connectivity and positive affect with antidepressant treatment in major depression. Am J Psychiat. 2013;170(2):197–206. doi: 10.1176/appi.ajp.2012.12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. P Natl Acad Sci USA. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiat. 2005;58(11):843–853. doi: 10.1016/j.biophysh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Keller J, Young CB, Kelley E, Prater K, Levitin DJ, Menon V. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J Psychiat Res. 2013;47(10):1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15(3):170–180. doi: 10.1038/nrn3666. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Von Cramon DY, Muller K, Friederici AD. Investigating emotion with music: An fMRI study. Hum Brain Mapp. 2006;27(3):239–250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Maess B, Grossmann T, Friederici AD. Electric brain responses reveal gender differences in music processing. Neuroreport. 2003;14(5):709–713. doi: 10.1097/01.wnr.0000065762.60383.67. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13(5):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Krumhansl CL. An exploratory study of musical emotions and psychophysiology. Can J Exp Psychol. 1997;51(4):336–353. doi: 10.1037/1196-1961.51.4.336. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsyc. 2005;27(3):320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Lepping RJ, Atchley RA, Chrysikou E, Martin LE, Clair AA, Ingram RE, Simmons WK, Savage CR. Neural processing of emotional musical and nonmusical stimuli in depression. PLOS One. 2016;11(6):23. doi: 10.1371/journal.pone.0156859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos AS, Gold C, Wang X, Crawford MJ. Music therapy for depression. Cochrane DB Syst Rev. 2008;1(CD004517) doi: 10.1002/14651858.CD004517.pub2. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Yurgelun-Todd D. Striatal structure and function in mood disorders: A comprehensive review. Bipolar Disord. 2010;12(8):764–785. doi: 10.1111/j.1399-5618.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. NeuroImage. 2005;28(1):175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Misaki M, Suzuki H, Savitz J, Drevets WC, Bodurka J. Individual variations in nucleus accumbens responses associated with major depressive disorder symptoms. Sci Rep-UK. 2016;6:12. doi: 10.1038/srep21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiat. 2011;70(4):395–399. doi: 10.1016/j.biopsych.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH. Psychometric evaluation of the Snaith-Hamilton pleasure scale in adult outpatients with major depressive disorder. Int Clin Psychopharm. 2010;25:328–333. doi: 10.1097/YIC.0b013e32833eb5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Bluhm RL, Williamson PC, Theberge J, Densmore M, Neufeld RWJ. Brain activation to favorite music in healthy controls and depressed patients. Neuroreport. 2009;20(13):1204–1208. doi: 10.1097/WNR.0b013e32832f4da3. [DOI] [PubMed] [Google Scholar]
- Pecina S. Opioid reward ‘liking’ and ‘wanting’ in the nucleus accumbens. Physiol Behav. 2008;94(5):675–680. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiat. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. P Natl Acad Sci USA. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. P Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi: 10.1017/s0033291713002535. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257–U355. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340(6129):216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- Sanders G, Wenmoth D. Verbal and music dichotic listening tasks reveal variations in functional cerebral asymmetry across the menstrual cycle that are phase and task dependent. Neuropsychologia. 1998;36(9):869–874. doi: 10.1016/s0028-3932(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE. Deep brain stimulation for major depression: Steps on a long and winding road. Biol Psychiat. 2015;78(4):218–219. doi: 10.1016/j.biopsych.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiat. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. P Natl Acad Sci USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone: The Snaith-Hamilton pleasure scale. Brit J Psychiat. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Aldi M, Bauer M, Heinz A, Strohle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26(5):677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Okamura N, Kawachi Y, Tashiro M, Arao H, Hoshishiba T, Gyoba J, Yanai K. Discrete cortical regions associated with the musical beauty of major and minor chords. Cogn Affect Behav Ne. 2008;8(2):126–131. doi: 10.3758/cabn.8.2.126. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav R. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage. 2009;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiat. 2016;6:e810. doi: 10.1038/tp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


