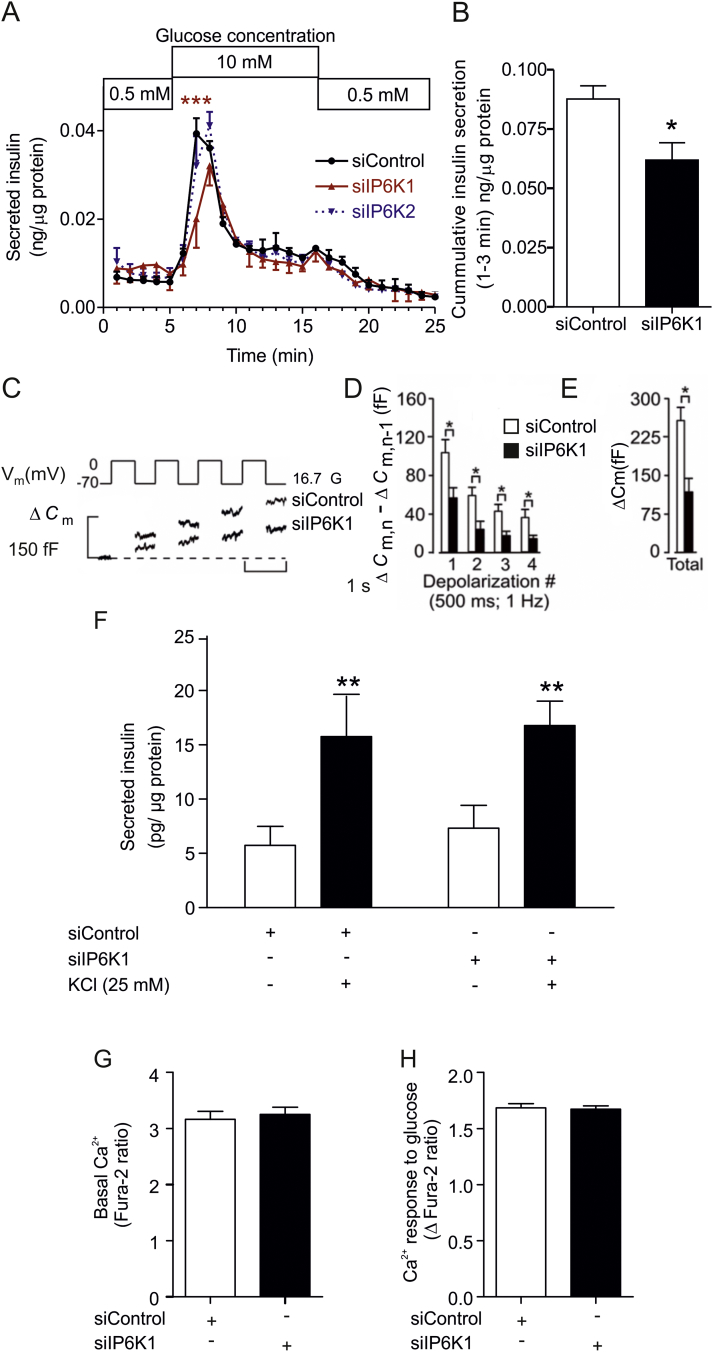
Fig. 2.
IP6K1 regulates first phase of glucose-stimulated exocytosis. (A) Effects of IP6K1 or IP6K2 siRNA on insulin release were studied using dynamic incubation assays in MIN6m9 cells. There was a significant difference in insulin release between control and IP6K1 siRNA after 2 min of glucose stimulation, n = 3, ***p < 0.001, two-way ANOVA (Supplemental Table S1). (B) Insulin secretion data from panel (A) were compiled for the first 3 min, n = 3, *p < 0.05, Student's t-test. (C) Mouse islet cells were transfected with fluorescently tagged siRNA to IP6K1 or a negative control. Fluorescent cells were subjected to a train of four 500-ms depolarizations (1 Hz) using the perforated patch configuration. Increases in cell capacitance (ΔCm) were measured at 16.7 mM glucose (16.7 G) in the extracellular medium. Recordings are representative of 5–8 experiments. (D) Histogram summarizing the average increment in cell capacitance per pulse (ΔCm,n − ΔCm,n−1) during the train of depolarization in cells transfected with either siRNA to IP6K1 or negative control, n = 5–8, *p < 0.05, Student's t-test. (E) Effect of siRNA to IP6K1 on total capacitance increase, n = 5–8, *p < 0.05, Student's t-test. (F) KCl mediated insulin secretion is not mediated via IP6K1. MIN6m9 cells treated with either control siRNA or IP6K1 siRNA were stimulated for 3 min with an addition of 25 mM KCl. There was a significant increase in insulin secretion in both control siRNA and IP6K1 siRNA treated group, n = 3, **p < 0.01, repeated measures two-way ANOVA followed by Bonferroni post-hoc test. (G) Basal [Ca2+]i and (H) glucose (10 mM) induced [Ca2+]i response were measured in control and IP6K1 siRNA-treated MIN6m9 cells (n = 3 preparations each compiled from 30 to 40 ROIs). All bars are means ± SEM. See experimental procedures for [Ca2+]i data analysis.