Figure 2. Enterohepatic circulation of bile acids under normal physiological conditions (a) and during dysbiosis and inflammation (b).
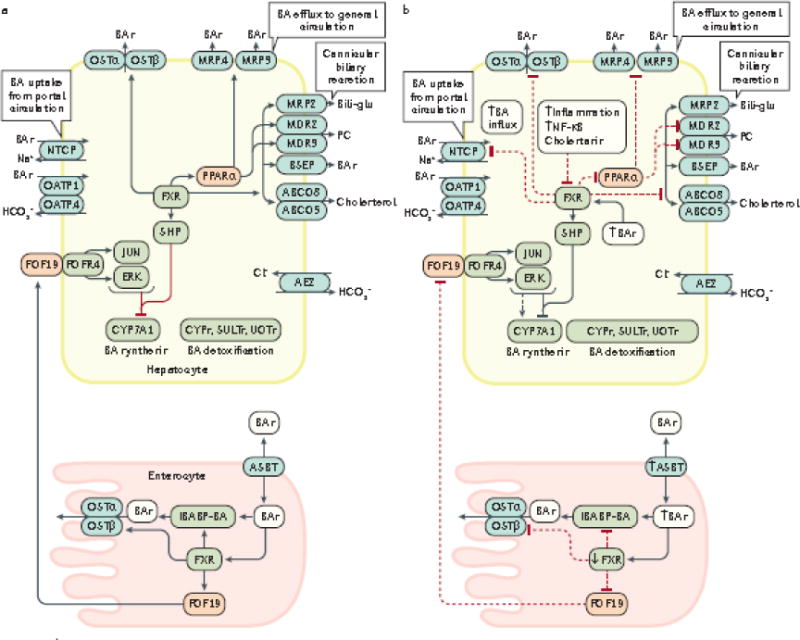
The enterohepatic circulation of bile acids between the intestine (enterocytes) and liver (hepatocytes) under normal physiological conditions (a, solid black arrows), and the alterations that occur in this process during dysbiosis of the gut microbiota and inflammation (b, dashed black arrows), are shown. During intestinal inflammation, which occurs as a result of intestinal barrier dysfunction, expression of intestinal FXR is downregulated207, which results in: reduced FGF19-FGFR4 signalling; downregulation of intestinal bile acid transport protein (IBABP); downregulation of the bile acid efflux transporters OSTα an OSTβ54,208; and upregulation of the apical sodium-dependent BA transporter (ASBT). Reduced expression of IBABP carrier protein results in a reduction in the transfer of bile acids across the enterocyte to the OSTα/β efflux transporters for entry into the portal vein, thus disrupting enterohepatic circulation. The combination of these events permit increased influx of bile acids into the enterocyte and prevention of bile acid efflux back into the portal vein. Collectively, increased influx and decreased efflux of bile acids might increase inflammation in the intestinal mucosa49. Diminished signalling via the FGF19-FGFR4 axis also increases bile acid synthesis in the liver via reduced activation of c-JUN/ERK/CYP7A1 axis209–211. During hepatic inflammation caused by bile acid perturbation of hepatocyte membranes and subsequent activation of pro-inflammatory PKC pathways leads to the activation NF-κB which inhibits transcription of hepatic FXR, and also prevents the subsequent activation of SHP that normally inhibits the synthesis of the rate-limiting enzyme for BA synthesis, CYP7A1. Therefore, decreased FXR expression promotes increased bile acid synthesis in combination with increased influx of bile acids98 due to the fact that OSTα/β, MRP3/4 and all of the cannicular transporters (depicted in grey ovals) are under transcriptional control by FXR.98 Decreased FXR transcription also leads to decreased expression of NTCP transporters which are involved in bile acid influx; however, OATP transporters are not affected by FXR212. FXR also controls bile acid detoxification; decreased transcription of FXR leads to decreased expression of PPARα and its target genes which encode Cytochrome P450 enzymes (CYPs), sulfotransferases (SULTs) and UDP-glucuronosyltransferases (UGTs).208 Decreased PPARα expression also induces additional decreases in expression of the MDR2/3, MRP3 and MRP4 transporters. Under these conditions, both cholestasis and inflammation are intensified which can lead to the development of liver cancer. Cholestasis causes inflammation via various mechanisms which leads to upregulation and activation of NF-κB which in turn, binds directly to the FXR promoter to inhibit its transcription124.
BSEP, bile salt export pump; CYP7A1, cholesterol-7α-hydroxylase; MDR3/4, multidrug resistance protein 3/4; MRP2/3/4, multidrug resistance-associated protein 2/3/4; NTCP, sodium taurochlorate co-transporting polypeptide; Ostα/β, organic solute transporter α/β; PC, phosphatidylcholine; PPARα, peroxisome proliferator-activated receptor α; SHP, small heterodimer partner; IBABP, ileal bile acid binding protein; FGF19, fibroblast growth factor 19; OATP, multi-specific orgnanic anion transporters; FGFR4, fibroblast growth factor receptor 4; ABCG5/8 ATP-binding cassette subfamily G, members 5/8; ASBT, apical sodium-dependent bile acid transporter; SULTs, sulfotransferases; UGTs, UDP-glucuronosyltransferases
