Figure 3. ERK regulates FOXC2‐dependent DLL4 expression in LPS‐treated HPMECs.
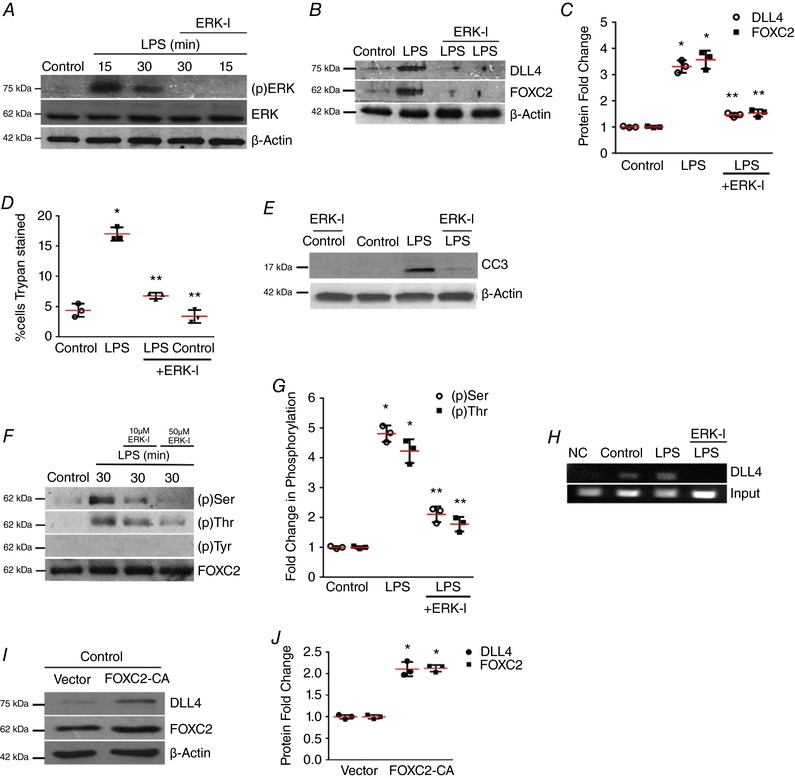
A, ERK phosphorylation [(p)ERK] was quantified at 15 and 30 min after LPS with or without ERK‐I pre‐treatment, n ≥ 3. B and C, DLL4 and FOXC2 protein were quantified at 24 h after LPS with or without ERK‐I, with densitometric quantification shown graphically (C), P < 0.01 (*Control vs. LPS; **LPS vs. ERK‐I + LPS), n = 3. D, HPMEC cell death (%) was quantified by Trypan Blue staining 24 h after LPS treatment with or without ERK‐I (50 μm), P < 0.01 (*Control vs. LPS: **LPS vs. ERK‐I + LPS), n = 3. E, CC3 protein level was assessed by immunoblotting after LPS treatment with or without ERK‐I. F, FOXC2 protein was immunoprecipitated from HPMECs at 30 min after LPS treatment with or without ERK‐I (10 and 50 μm), and serine, threonine and tyrosine phosphorylation quantified by densitometry (G), P < 0.001 (*Control vs. LPS: **LPS vs. ERK‐I + LPS), n = 3. H, HPMEC nuclear lysates obtained 30 min after LPS with or without ERK‐I treatments were used to quantify FOXC2 binding to the DLL4 promoter by ChIP, n ≥ 3. I, DLL4 and FOXC2 protein were assessed after transfection with FOXC2‐CA (CA, constitutively active) or empty plasmid, and quantified by densitometry (J), * P < 0.01 (Control vs. FOXC2‐CA), n = 3. [Color figure can be viewed at http://wileyonlinelibrary.com]
