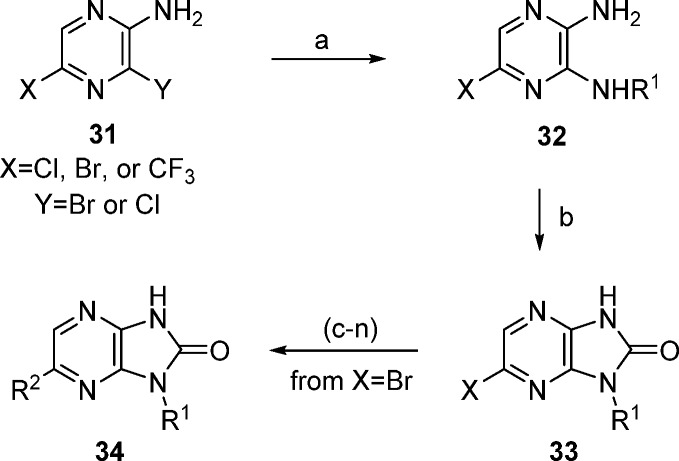
Scheme 1. Synthesis of Imidazopyrazinone Derivatives.
Reagents and conditions: (a) NHR1. (b) CDI, THF. (c) (R2 = furyl or phenyl) R2B(OH)3, Cl2Pd(dppf), Na2CO3, dioxane/water. (d) (R2=SMe) NaSMe, NMP. (e) (R2 = OMe) NaOMe, NMP. (f) (R2 = CN) Zn(CN)2, (Ph3P)4Pd, DMF. (g) (R2 = Ac) tributyl(1-ethoxyvinyl)tin, Cl2Pd(dppf), dioxane. (h) (R2 = H) H2, Pd/C, EtOH. (i) (R2 = Me) trimethylboroxine, Cl2Pd(dppf), Na2CO3, dioxane/water. (j) (R2 = H3CC≡C) H3CC≡CH, Cl2Pd(dppf), CuI, TEA, dioxane/water. (k) (R2 = HC≡C). (l) 1. TMSC≡CH, Cl2Pd(dppf), CuI, TEA, dioxane/water; 2. KF, MeOH/THF/water. (m) (R2 = vinyl) trimethylboroxine, Cl2Pd(dppf), Na2CO3, dioxane/water. (n) (R2 = Et) from R2 = vinyl; H2, Pd/C, EtOH.