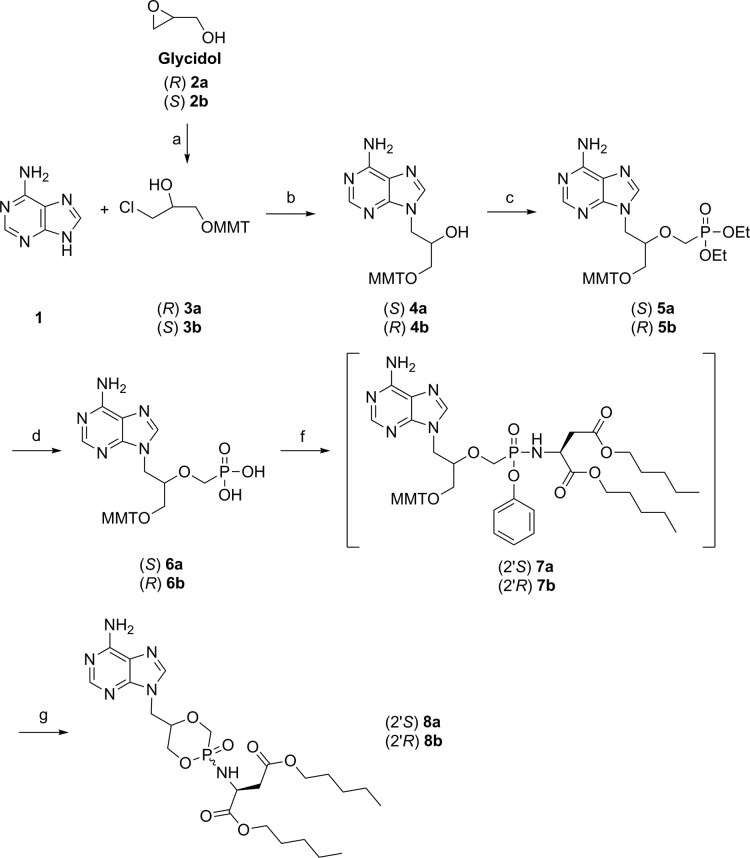
Scheme 1. Synthesis of (S)- and (R)-cHPMPA l-Asp-diamylphosphonamidates 8a and 8b.
Reagents and conditions: (a) MMTCl, Pyr, 50 °C, 20 h, 80%; (b) NaH, DMF, 110 °C, 6 h, 40–50%; (c) diethyl tosyloxymethylphosphonate, NaH, THF, 0 °C to rt, 5 h, 35–40%; (d) TMSBr, 2,6-lutidine, CH3CN, 0 °C to rt, 12 h, 70%; (f) (i) l-aspartic acid amyl ester HCl salt, PhOH, 2,2′-dithiodipyridine, PPh3, Et3N, Pyr, 60 °C, 12 h; (g) TCA (6% in DCM), rt, 2 h, 37–42% over 2 steps.