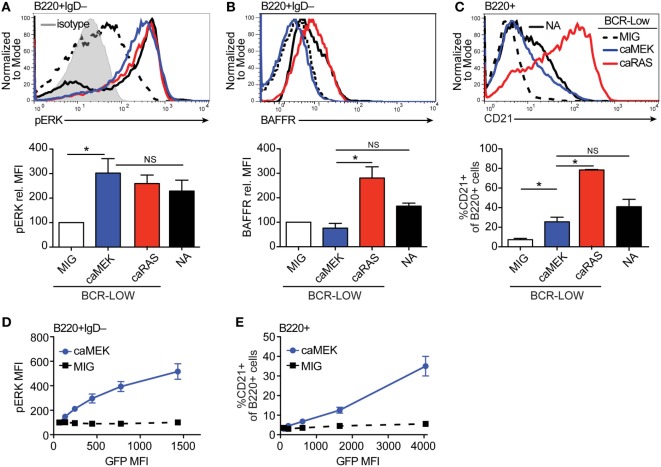
Figure 2.
Extracellular signal-regulated kinase (ERK) activation drives differentiation of BCR-low immature B cells in vitro. (A,B) Representative histograms and bar graph quantification of pERK (A) and BAFFR (B) in bone marrow immature (B220+IgD–) B cells cultured for 4 days with IL-7. The cells analyzed were either nonautoreactive (NA) (3-83Igi,H-2d) BCR normal cells (NA, black solid line) or BCR-low cells transduced with MIG (black dashed line), caMEK (blue line), or caNRas (red line). The analysis of transduced cells was performed on GFP+ cells in all experiments. Staining of pERK was done on cells treated with pervanadate to allow for pERK signal detection. The gray shaded histogram in (A) represents isotype control antibody. (C) Representative histograms and bar graph quantification of the frequency of CD21+ cells in the B220+ B cell population after 3 days of culture with BAFF. (D,E) BCR-low B cells transduced with either caMEK (blue) or MIG control (black) were gated based on increasing GFP expression, which correlates with caMEK expression in caMEK transduced cells. The pERK MFI (D) or the frequency of CD21+ cells (E) were plotted against the GFP MFI of the individual segments. In all panels, N = 3 total, from three independent experiments. *P ≤ 0.05; NS, not significant.