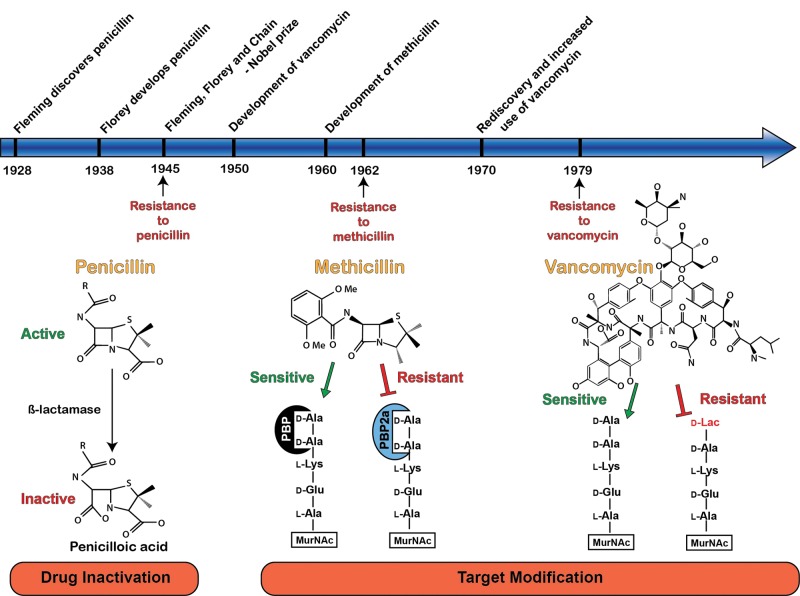
Figure 1. Timeline of selected antibiotic development and reported resistance.
A mechanism of resistance is illustrated for each antibiotic. Penicillin is commonly inactivated by bacterial β-lactamases, which cleave the β-lactam ring, forming the inactive penicilloic acid. Subsequent development of methicillin utilized a larger aryl side chain that was largely resistant to hydrolytic cleavage by β-lactamases. Instead, resistance to methicillin is driven by the expression of the alternative transpeptidase, PBP2a, which has a lower affinity for methicillin and can catalyse peptidoglycan cross-linking despite methicillin intervention. Resistance to vancomycin is driven by structural alteration of the terminal dipeptide that is modified from d-alanyl-d-alanine (d-Ala-d-Ala) to d-alanyl-d-lactate (d-Ala-d-Lac), reducing the affinity of the dipeptide for vancomycin and preventing disruption of peptidoglycan cross-linking.