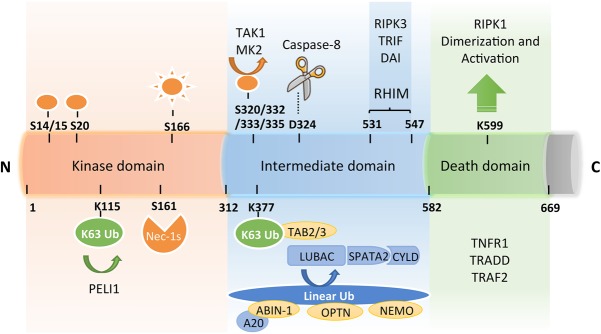
Figure 1.
A schematic diagram of RIPK1 domains, interacting proteins, post-translational modifications, and the catalytic enzymes that read or write these modifications. RIPK1 contains an N-terminal kinase domain, an intermediate domain with a RIP homotypic interaction motif (RHIM), and a C-terminal DD. The phosphorylation and ubiquitination sites as well as types of ubiquitin linkages on RIPK1 are indicated together with their respective catalytic enzymes. K63 ubiquitination chains on Lys377 mediate the recruitment of TAB2/3 and the activation of transforming growth factor-β-activated kinase 1 (TAK1), which in turn phosphorylates IKKs for activating the NF-κB pathway. M1 ubiquitination of RIPK1 is regulated by the linear ubiquitination assembly complex (LUBAC) and the deubiqutinating complex CYLD/SPATA2. The ubiquitin-binding proteins ABIN-1, Optineurin (OPTN), and NEMO, each carrying a UBAN motif that can bind with the linear ubiquitination chains, play important roles in regulating the activation of RIPK1. The RHIM motif of RIPK1 is required for binding with RIPK3 to mediate necroptosis and may also interact with TRIF, DAI, and Toll-like receptors to promote inflammation. Autophosphorylation of Ser166 is a biomarker for RIPK1 activation. The small molecule Nec-1s is caged in a hydrophobic pocket between the N and C lobes of the kinase domain and forms an H bond between its nitrogen atom and the hydroxyl oxygen of Ser161 on the activation loop to inhibit the activation of RIPK1. The cleavage of RIPK1 after Asp324 by caspase-8 or the phosphorylation on Ser320/331/333/335 in human RIPK1 and Ser321/332/334/336 in murine RIPK1 by TAK1 or MK2 leads to the suppression of RIPK1 activation. E3 ligase Pellino 1 (PELI1) mediates K63 ubiquitination on Lys115 of activated RIPK1 to promote complex IIb formation and necroptosis. The DD is not only crucial for the initiation of TNFR1 signaling but also indispensable for RIPK1 activation by mediating RIPK1 dimerization during the transition from complex I to complex II.