Figure 2.
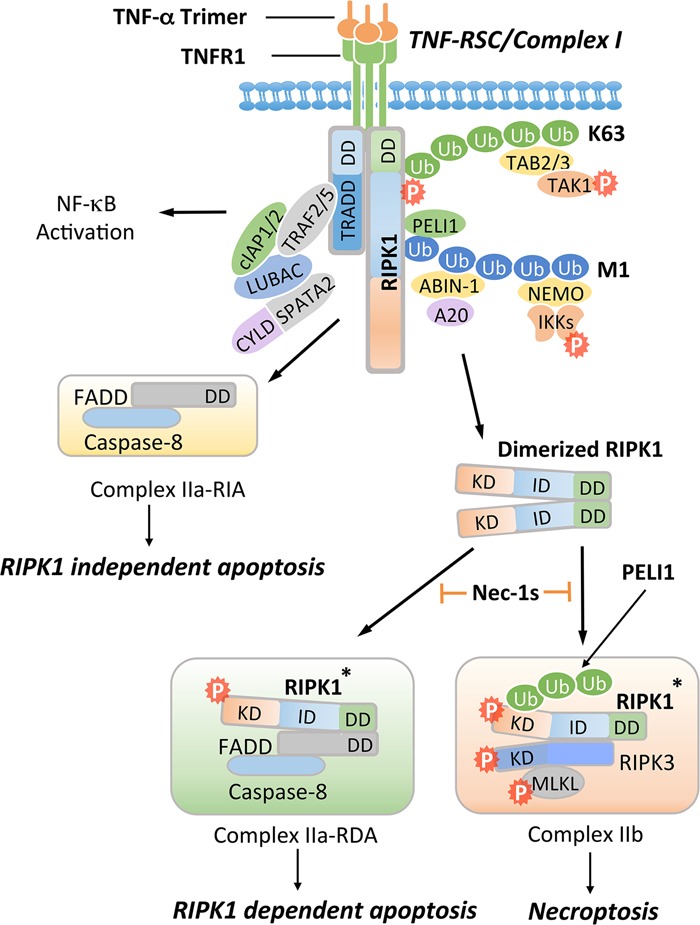
Activation of TNFR1 may promote multiple alternative signaling pathways, including the activation of NF-κB, RIPK1-independent apoptosis (RIA), RDA, and necroptosis. Activation of TNFR1 by trimerized TNFα induces the formation of a membrane-associated transient complex (named complex I or TNF-RSC), which includes RIPK1, TRADD, TRAF2/5, cIAP1/2, LUBAC, ABIN-1, A20, NEMO, PELI1, CYLD, and SPATA2. In complex I, RIPK1 is rapidly polyubiquitinated by K63-linked and M1-linked ubiquitin chains mediated by cIAP1/2 and LUBAC, respectively. K63 ubiquitination chains on RIPK1 mediate the recruitment of TAB2/3 to facilitate the activation of TAK1, leading to phosphorylation of the IKK complex and RIPK1 on S320/S331/S333/S335. M1 ubiquitination chains on RIPK1 mediate the recruitment of PELI1, ABIN-1, and NEMO. ABIN-1 in turn recruits phosphorylated A20 to promote K63 deubiquitination of RIPK1. CYLD/SPATA2 promote the M1 deubiquitination of RIPK1. The activated IKK/NEMO complex promotes the activation of NF-κB. When the output of the NF-κB pathway is inhibited by cycloheximide, which blocks the expression of cFLIPL, FADD interacts with caspase-8 to form complex IIa-RIA to promote RIA. Alternatively, dimerization of RIPK1 can mediate the activation of RIPK1 during the transition from complex I to complex II. Activated RIPK1* can interact with FADD and caspase-8 to form complex IIa-RDA to promote RDA. When caspase-8 is inhibited, activated RIPK1* is ubiquitinated by PELI1 on K115 and binds with RIPK3 to form complex IIb, leading to MLKL activation and necroptosis. Nec-1s inhibits the activation of RIPK1 kinase and in turn prevents the formation of complex IIa to block RDA and complex IIb to block necroptosis.
