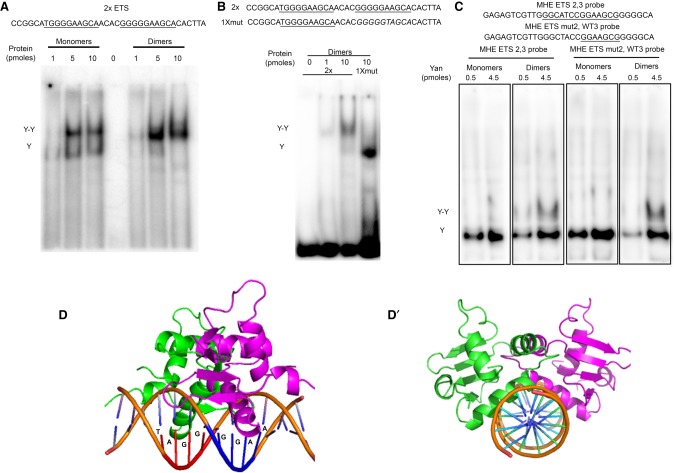
Figure 4.
The overlapping anti-parallel configuration of the ETS2,3 pair may preclude classic cooperative recruitment of Yan dimers. (A–C) Gel shifts using 32P-labeled probes and recombinant Yan monomers (YanA86D) or Yan dimers (1:1 YanA86D:YanV105R). Increasing concentrations of total Yan protein are indicated above each gel. (A) SAM-mediated dimerization induces classic cooperative binding to probe with two consensus ETS sites (2× ETS). Single-bound (Y) and double-bound (Y–Y) species are indicated. (B) Cooperative binding requires two ETS sites. (C) The MHE ETS2,3 sites do not support simultaneous occupancy by two Yan molecules. The slower-migrating species (Y–Y) observed with Yan dimers occurs even when one ETS site is mutated, suggesting that it reflects association of the second Yan molecule to an adjacent nonspecific sequence. (D) A structural model of a Yan ETS DNA-binding domain (DBD) dimer in complex with the MHE ETS2,3 sequence, viewed from the side (D) and along the axis of the DNA (D′), predicts a strong steric clash that should preclude simultaneous occupancy of both ETS sites. The two Yan DBDs are colored in green and purple.