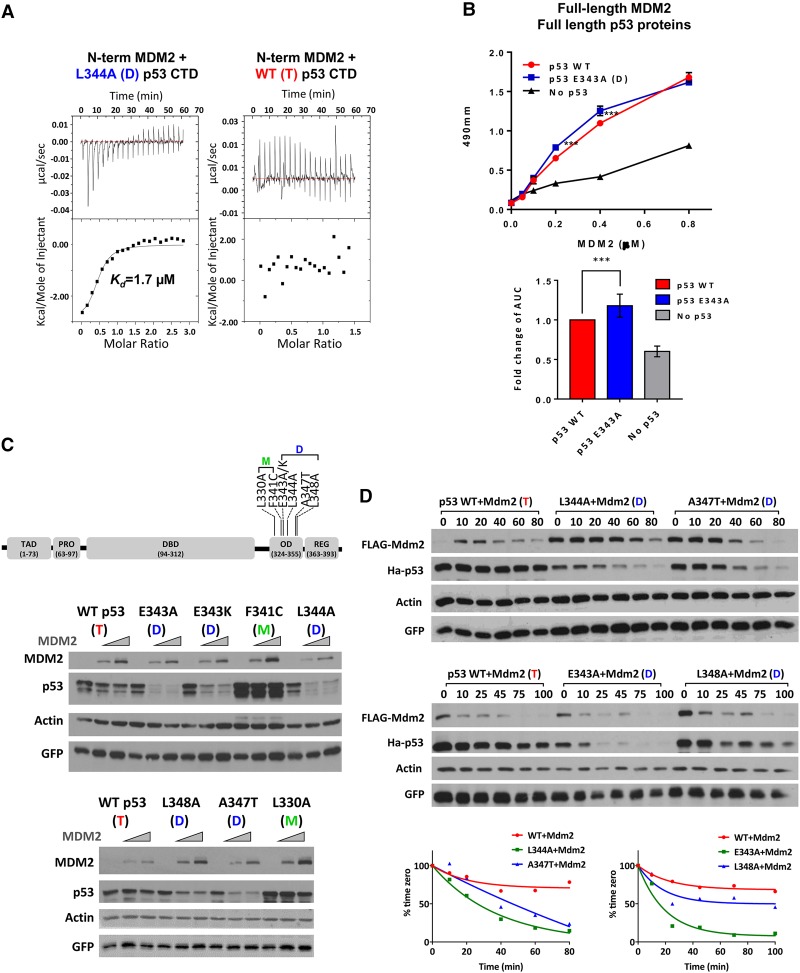
Figure 1.
MDM2 preferentially binds and degrades dimer-forming mutant versions of p53. (A) Polypeptides from wild-type or L344A p53 CTDs (residues 293–393; 0.5 mM) were titrated into a solution containing 0.02 mM an MDM2 NTD polypeptide (residues 10–139), and their interactions were measured by ITC as described in the Materials and Methods. The MDM2 NTD bound the mutant p53 CTD (L344A) with an affinity of 1.7 µM. (B) The top panel shows a binding curve of p53 to MDM2 from one representative experiment. Purified full-length wild-type or E343K mutant p53 (0.03 mM) proteins were used to coat ELISA plates followed by addition of full-length purified wild-type MDM2 protein at the indicated concentrations. Complexes were detected using anti-MDM2 antibody (N-20) and enzyme-conjugated secondary antibody as described in the Materials and Methods. The bottom panel graphically depicts the cumulative binding data from six replicative experiments. The data represent the mean ± SEM for six biological replicates with two technical replicates each. (***) P-value = 0.0002, calculated by nonparametric one-way ANOVA as described in the Materials and Methods. (C, top panel) A diagram of a p53 protein showing the TAD (residues 20–60), proline domain (Pro; residues 63–97), DBD (residues 94–312), OD (residues 324–355), and C-terminal REG (residues 363–393). The positions of the dimer-forming (D) and monomer-forming (M) mutations are indicated. The two panels below the diagram are typical experiments showing results of transfecting U2OS cells with increasing amounts of Flag-MDM2 (0, 375, and 750 ng) and a constant amount of HA-tagged wild-type or OD mutant versions of p53 (150 ng) as indicated. Twenty-four hours later, cells were harvested, and lysates were used for immunoblotting with the indicated antibodies. (D) U2OS cells were transfected with 1.2 µg of Flag-MDM2 and 150 ng of HA-tagged wild-type or OD mutant p53, as indicated. Twenty-four hours after transfection, 100 µg/mL cycloheximide (CHX) was added, and cells were harvested at the indicated times. (Top panels) Cell lysates were subjected to immunoblotting with the indicated antibodies. (Bottom panels) Quantification of the immunoblotting data was carried out using ImageJ software.