Figure 1.
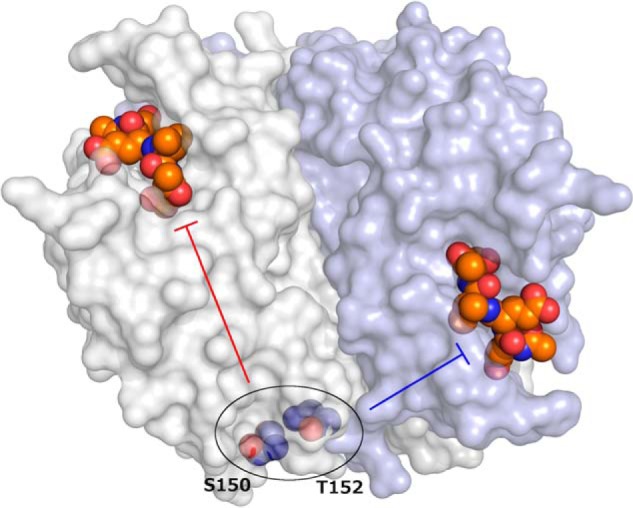
Caspase-3 catalytic activity can be allosterically modulated by phosphorylation at Ser150 or Thr152. The caspase-3 dimer (Protein Data Bank code 2J30) is illustrated with one protomer shown as a white surface and the other as a light blue surface. Active sites are shown with the Ac-DEVD-CMK inhibitor as colored spheres, with orange carbon atoms. Conformational changes that occur upon phosphorylation of Ser150 or Thr152 (colored spheres with blue carbon atoms) are propagated to the active sites through distinct intraprotomer (red bar-headed arrow) or interprotomer (blue bar-headed arrow) pathways.
