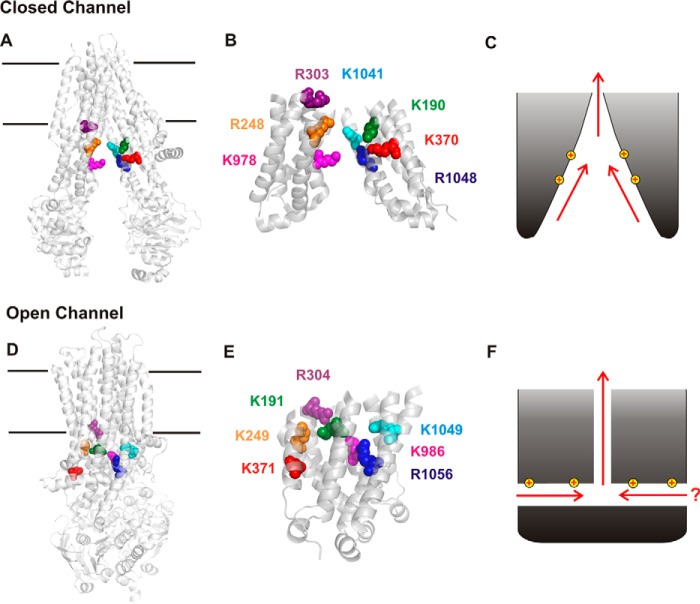
Figure 1.
Structural and functional models of the cytoplasmic entrance to the CFTR channel pore. A, overall structure of human CFTR in the channel closed state (7). In this state, the TMs and TMEs are arranged in an “inward facing” conformation, such that the transmembrane permeation pathway is open to the cytoplasm and closed at its extracellular end. Those positively charged amino acid side chains that have been proposed, based on functional data, to attract cytoplasmic Cl− ions to the permeation pathway are shown as space-filling models. These positive charges contribute to the overall positive electrostatic potential of this part of the protein (8). B, close-up view of the isolated TMEs (derived from A), showing the location of these positively charged side chains as labeled in the same colors. C, the wide-open nature of the TMEs suggests that Cl− and other substances could be “funneled” toward the transmembrane pore in this closed state. D, overall structure of zebrafish CFTR in a near-open state (8). In this state, the TMEs are much more closely associated, and there is no “central” pathway from the cytoplasm to the pore (such as apparently exists in A). Positively charged amino acid side chains corresponding to those in A are shown using the same color scheme. E, close-up view of the isolated TMEs (derived from D). Note that the numbering of these positively charged amino acids is slightly different in zebrafish CFTR. F, tight closure of the inner ends of the TMEs led us to suggest that cytoplasmic Cl− accesses the open channel pore by at least one, and possibly two, cytoplasmic portals that are lined by positively charged amino acid side chains that play an important role in electrostatic attraction of Cl− to the permeation pathway (17).