Figure 3.
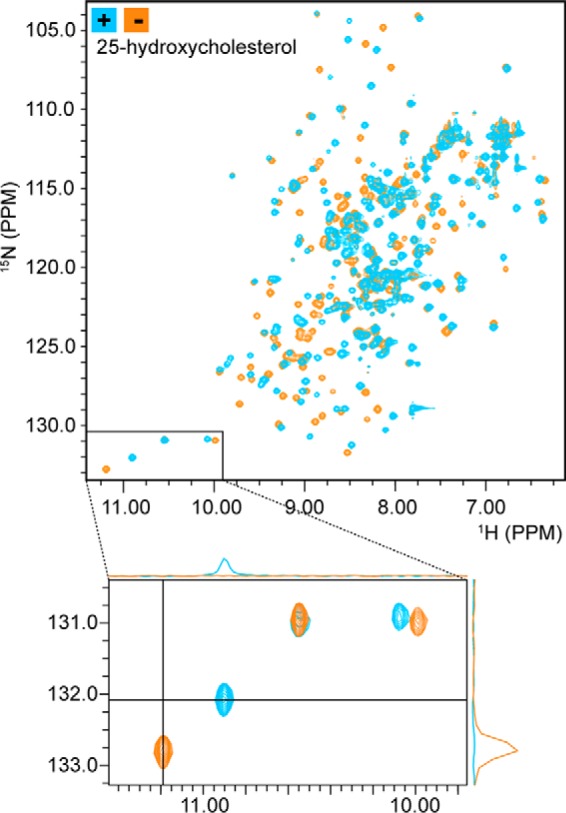
NMR spectroscopy reveals sterol binding by Lam4S2. 2D 1H-15N correlation spectra (HSQC) of Lam4S2 with and without bound sterol are shown. The spectra are well dispersed, consistent with an intact secondary and tertiary structure. Comparing spectra acquired in the absence (orange) and presence (blue) of 25-hydroxycholesterol reveals a large number of chemical shift changes, similar to those observed for other StART domains upon ligand binding and consistent with quantitative binding of 25-hydroxycholesterol. The bottom panel is an enlargement of the lower left region of the 2D HSQC spectrum, showing that resonances that shift upon sterol binding do so completely with no residual intensity at the location of the original signals. 1D projections further demonstrate the absence of free-state signals in the bound-state spectrum (upper projection) and the absence of bound-state signals in the free-state spectrum (right-hand projection).
