Figure 4.
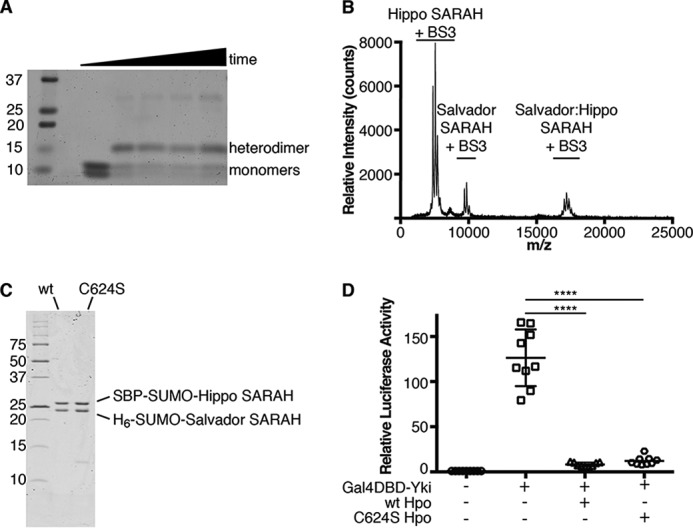
Salvador–Hippo SARAH is a heterodimer. A, SDS-PAGE gel of cross-linking reactions containing Salvador and Hippo SARAH domains and BS3 at 0, 30, 60, 120, or 180 min. Initially the two SARAH domains run as monomers, and a second band appears at 13 kDa corresponding to the heterodimer during incubation with BS3 and remains present throughout the time course. B, MALDI-MS spectrum of the cross-linking reaction at the 30-min time point. This spectrum shows that the 17-kDa species matches a Salvador–Hippo SARAH heterodimer rather than either possible homodimer. The samples taken for analysis using SDS-PAGE and MALDI-MS came from the same reaction. The difference in apparent abundance between the gel image and the mass spectrum is caused by the difference in ionizability between the different protein species. C, SDS-PAGE following IMAC purification of tagged Salvador–Hippo SARAH complexes containing either WT (wt) or C624S Hippo SARAH domains (C624S). D, S2 cells were transfected with plasmids encoding Renilla, a Gal4 responsive luciferase reporter (white circle), Yorkie fused to the Gal4 DNA-binding domain (Gal4DBD-Yki) (white square), and either wt (white triangle) or C624S Hippo (white hexagon). After 3 days, the levels of luciferase were measured. Each measurement was normalized to the signal from Renilla luciferase. The data were plotted as the relative firefly luciferase signal compared with that from Gal4DBD-Yki and are from nine measurements from four independent experiments, the mean is indicated by a horizontal bar with error bars corresponding to the S.D. among measurements. p value ≤ 0.0001, compared with control. The p value between wt and C624S Hippo is 0.8906.
