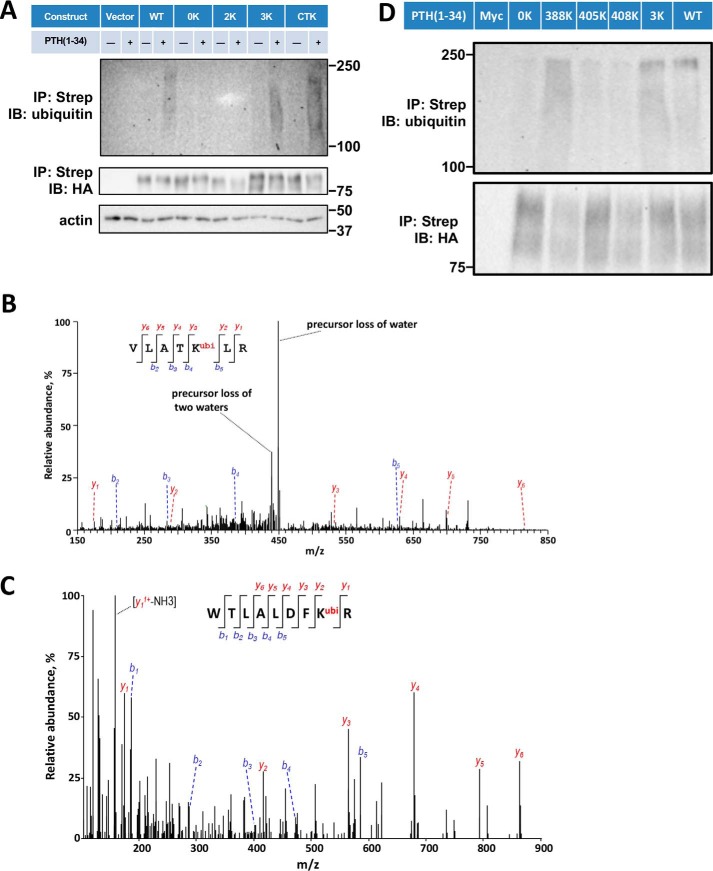
Figure 3.
Sites of PTHR ubiquitination. A, ubiquitination of WT-PTHR and the indicated 0K, 2K, 3K, and CTK-PTHR mutants. GnTI− cells stably expressing the specified PTHR were transfected with Myc-ubiquitin. After 48 h, cells were serum-starved for 2 h and treated with 10 μm MG132 for 30 min followed by a 30-min exposure to 100 nm PTH(1–34). Empty TAP-PTHR vector was transfected as a negative control. PTHR was isolated on streptavidin-agarose beads. The bound receptor was separated by SDS-PAGE and immunoblotted with antibodies specific to ubiquitin (P4D1) (top) and HA for receptor expression (middle). β-Actin was used as the loading control (bottom). Molecular weights are indicated at the right. Shown is a representative blot of three experiments. B and C, MS/MS spectra for the identified ubiquitinated peptides containing Lys388 or Lys484. The peptide sequences are shown at the top of the MS/MS spectra with ubiquitinated residues highlighted in red. Peak heights indicate the relative abundance of the corresponding fragmentation ions, with the annotation of the identified, matched N terminus–containing ions (b ion) in blue and C terminus–containing ions (y ions) in red. The spectra are representative of three independent MS/MS experiments. D, immunoblot detection of ubiquitination of loop 3 Lys mutants. Cells were transiently transfected with WT-PTHR or mutant PTHR and Myc-ubiquitin using Lipofectamine 3000. GnTI− cells transfected with Myc-ubiquitin (Myc) alone were used as a negative control. PTHR was isolated and detected by immunoblotting using antibodies directed against ubiquitin (P4D1) (top) and HA for PTHR (bottom). Molecular weights are indicated at the left. Experiments were repeated three times with similar results.