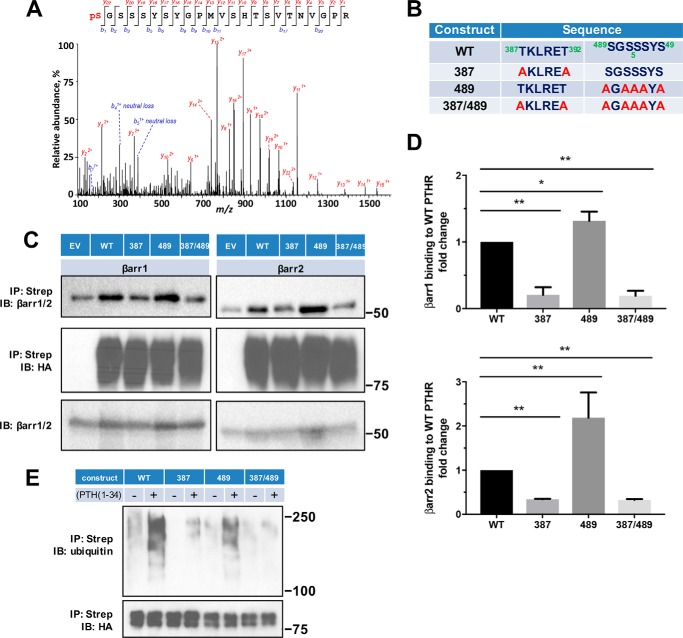
Figure 5.
β-Arrestin recruitment requires PTHR phosphorylation. A, representative MS/MS spectrum showing phosphorylated Ser489 (pS, red) in purified TAP(HA)-PTHR overexpressed in HEK-293S GnTI− cells. The peptide sequence corresponding to the MS/MS spectrum is shown at the top, with the phosphorylated residue highlighted in red. Peak heights indicate the relative abundance of the corresponding fragmentation ions. The identified matched N terminus–containing ions (b ion) in blue and C terminus–containing ions (y ions) in red are annotated. The experiment was repeated in triplicate with identical results. B, summary of constructs with their respective sequences for WT and mutant PTHR to define loop 3 phosphorylation sites. In the 387 construct, Thr387 and Thr392 were replaced by Ala (A). In the 489 construct, all Ser residues were changed to Ala. The 387/489 double mutant construct has all Thr and Ser residues in both regions replaced by Ala. C, representative immunoblots showing FLAG-β-arrestin1 (left) and FLAG-β-arrestin2 (right) from immunoprecipitated WT-, 387-, 489-, or 387/489-TAP(HA)-PTHR. After pulldown by streptavidin beads, protein samples were analyzed by Western blot with antibodies specific to β-arrestin1/2 (top) and HA for receptor expression (middle). Expression of β-arrestin1 or -2 in cell lysates is shown in the bottom panel. Molecular weights are indicated at the right. Note that some β-arrestin1/2 background was evident with empty vector (EV) despite HA-PTHR being undetectable. This background was subtracted during the subsequent quantification. Experiments were repeated three times with similar outcomes. D, quantitative summary of β-arrestin1 (top) or β-arrestin2 (bottom) binding to WT-PTHR of results shown in C. Data are means ± S.D. (error bars) of n = 3 independent observations for each group. Binding of β-arrestin to WT-PTHR was set as 1 (*, p < 0.05; **, p < 0.01 by ANOVA with post hoc analysis). E, effect of site-specific Thr/Ser mutation on PTHR ubiquitination. WT-PTHR or the indicated Ser/Thr mutant PTHR construct shown in B, together with Myc-ubiquitin, were transfected into HEK-293S GnTI− cells using Lipofectamine 3000. After 48 h, cells were serum-starved for 2 h and treated with 10 μm MG132 for 30 min, followed by a 30-min challenge with saline vehicle or 100 nm PTH(1–34). Ubiquitinated TAP-PTHR was pulled down with streptavidin beads and probed with an anti-ubiquitin antibody (P4D1, top) and HA for receptor expression (bottom). Molecular weight size markers are shown at the right. The 387 construct, where Thr387 and Thr392 were replaced by Ala, decreased but did not eliminate PTH-induced ubiquitination. In contrast, the 489 construct lacking all five Ser residues, did not affect receptor ubiquitination. The sample shown is illustrative of three experiments.