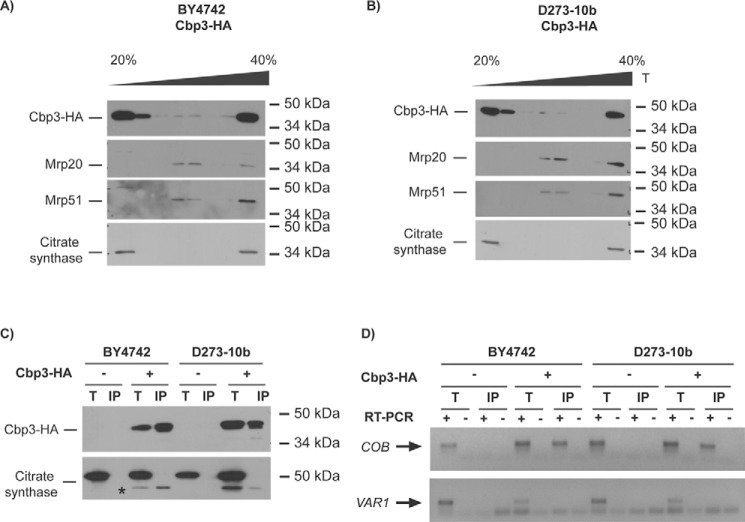
Figure 5.
Cbp3 and Cbp6 interact with the mitochondrial ribosome and with the COB mRNA in both D273-10b and BY4742 lab strains. A and B, 500 μg of isolated mitochondria from BY4742 (A) and D273-10b (B) cells expressing CBP3-HA from a low-copy plasmid were solubilized with digitonin, clarified, and loaded into a discontinuous sucrose gradient (20–40%) for ultracentrifugation. 1/10 of the clarified portion was taken as a total control (T). Proteins from seven fractions were TCA-precipitated and analyzed by SDS-PAGE (12%) and Western blotting. Cbp3–HA was detected with an antibody against the HA epitope. Antibodies against Mrp20 and Mrp51 were used as markers for large and small ribosomal subunits, respectively. Citrate synthase was used as a control for a non-ribosomal mitochondrial protein. C, 500 μg from isolated mitochondria were solubilized with dodecyl maltoside, and Cbp3–HA or untagged Cbp3 were subjected to immunoprecipitation with an antibody against HA. Immunoprecipitate (IP, one-third) and total (T, 5% of the mitochondrial extract used for immunoprecipitation) fractions were separated by SDS-PAGE and analyzed by Western blotting with antibodies against HA and citrate synthase (as a negative control for interaction). The asterisk corresponds to the previous HA immunoblot analysis of Cbp3–HA. D, RNA was extracted from the total (T) and immunoprecipitate (IP) fractions. Each fraction was divided in two, and cDNA was prepared in the presence (+) or absence (−) of reverse transcriptase (RT) using primers for the COB and VAR1 5′-UTRs. The (−) RT lanes represent a negative control for DNA contamination. The PCR products were run on agarose gel, and the gel pictures were color-inverted.