Figure 4.
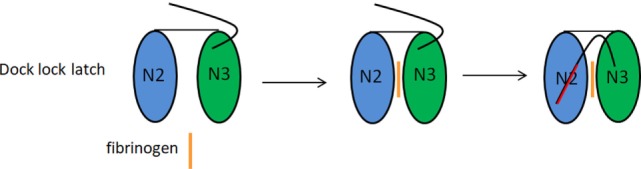
Fibrinogen binding by serine-rich repeat proteins (Srr) 1 and Srr2 by dock, lock, and latch (DLL) mechanism. The DLL mechanism is based on the observation that Srr1 and Srr2 proteins contain two subdomains, N2 and N3. The N2 and N3 subdomains concur to create a wide trench in which resides the ligand binding site. According the DLL mechanism the ligand (FBG) binding takes place in multiple steps: the ligand region first docks into the trench, followed by conformational change and redirection of the unstructured carboxy-terminal extension of the N3 subdomain, so that amino acid residues in this extension crossover the binding trench and lock the bound ligand in place. In the final latching event, the complex is further stabilized by the insertion of the N3 C-terminus through a β-strand complementation in a trench on the surface of the N2 subdomain.
