Figure 5.
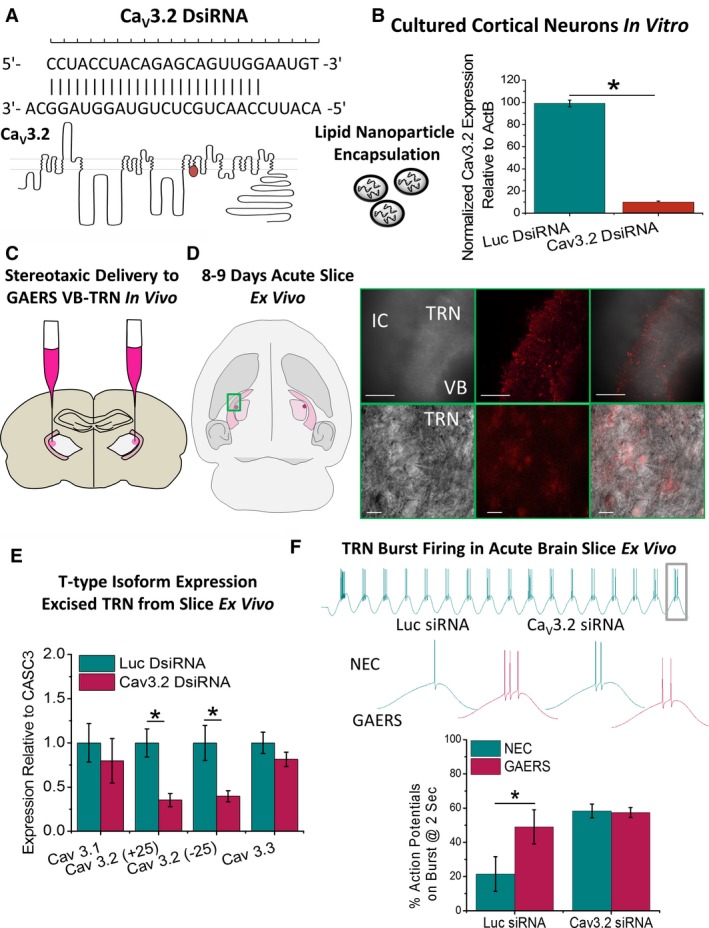
Selective knock‐down of thalamic Cav3.2 in vivo. A, Dicer siRNA (DsiRNA) was designed to selectively anneal to the Cav3.2 channel and target for degradation. DsiRNA was packaged with DiI into LNPs for ApoE‐mediated delivery to brain cells. B, Selective Cav3.2 knock‐down was first confirmed in vitro by applying to cultured cortical neurons for 48 hours and expression levels assessed with qPCR. C, For in vivo analyses, P120‐P150 GAERS rats were stereotaxically injected with luciferase control (Luc) or Cav3.2 DsiRNA LNPs bilaterally into the ventroposterior lateral thalamus to deliver DsiRNA to TRN neurons without damaging or lesioning the TRN. D, Following a 7‐ to 8‐day recovery period, animals were euthanized, the brain removed, and LNP invasion of TRN was confirmed by DiI fluorescence ex vivo (scale bars: top panels = 400 μm, bottom panels = 20 μm). E, Cav3.1‐Cav3.3 T‐type isoform expression in TRN tissue was assayed using qPCR relative to the control gene CASC3. Histograms display mean data for expression of T‐type calcium channel isoforms in TRN tissue dissected from 3 sequential 300‐μm‐thick horizontal brain slices for GAERS injected with Luc (n = 6 animals) and Cav3.2 DsiRNA (n = 8 animals); CaV3.1 isoform: Luc DsiRNA = 1.00 ± 0.22, CaV3.2 DsiRNA = 0.79 ± 0.25, P = .55 t test; CaV3.2(+25) splice variant: Luc DsiRNA = 1.00 ± 0.16, CaV3.2 DsiRNA = 0.35 ± 0.07, P = .0017 t test; CaV3.2(−25) splice variant: Luc DsiRNA = 1.00 ± 0.19, CaV3.2 DsiRNA = 0.39 ± 0.06, P = .0067 t test; CaV3.3 isoform: Luc DsiRNA = 1.00 ± 0.12, CaV3.2 DsiRNA = 0.81 ± 0.08, P = .21 t test. F, Oscillatory burst‐firing was assessed in TRN neurons from acute thalamic brain slices in P19‐P20 NEC and GAERS, 6‐8 d following injection with either Luc (n = 3 animals, n = 6 cells) or Cav3.2 (n = 3 animals, n = 7 cells) siRNA. Bar charts show percentage number of action potentials on the last burst relative to the first burst from a 2‐s oscillating wave stimulation at 8 Hz. *P < .05
